The Skinny on Fat and Covid-19
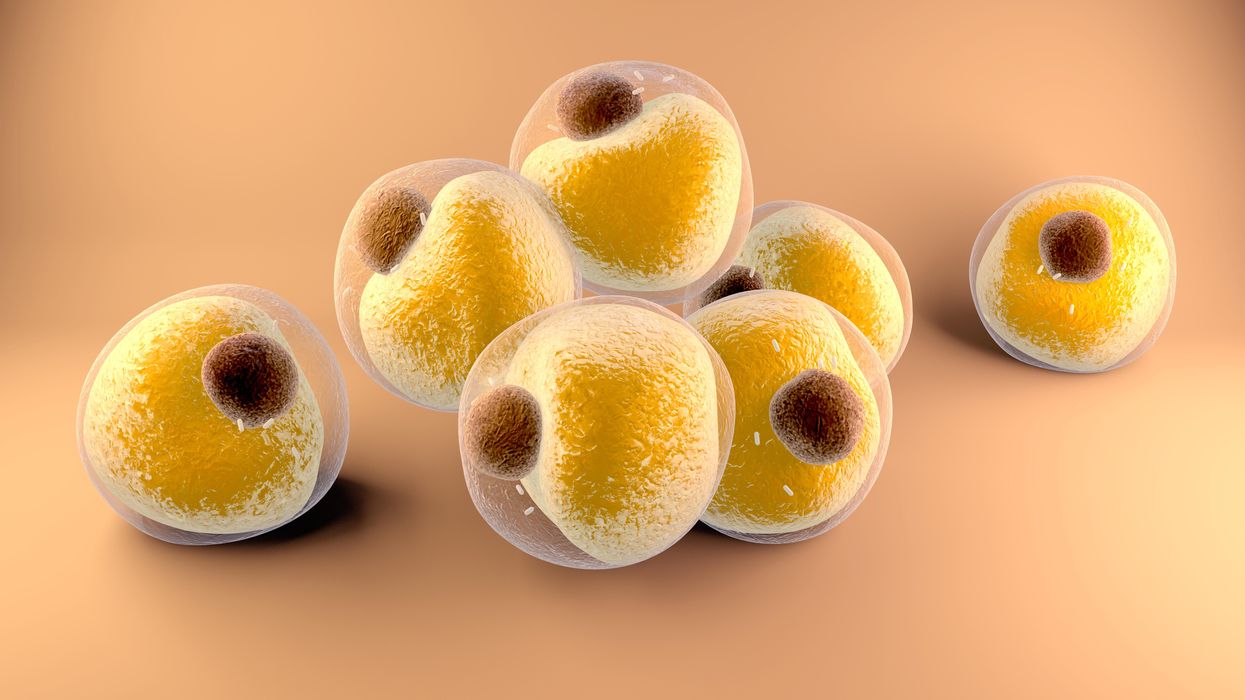
Researchers at Stanford have found that the virus that causes Covid-19 can infect fat cells, which could help explain why obesity is linked to worse outcomes for those who catch Covid-19.
Obesity is a risk factor for worse outcomes for a variety of medical conditions ranging from cancer to Covid-19. Most experts attribute it simply to underlying low-grade inflammation and added weight that make breathing more difficult.
Now researchers have found a more direct reason: SARS-CoV-2, the virus that causes Covid-19, can infect adipocytes, more commonly known as fat cells, and macrophages, immune cells that are part of the broader matrix of cells that support fat tissue. Stanford University researchers Catherine Blish and Tracey McLaughlin are senior authors of the study.
Most of us think of fat as the spare tire that can accumulate around the middle as we age, but fat also is present closer to most internal organs. McLaughlin's research has focused on epicardial fat, “which sits right on top of the heart with no physical barrier at all,” she says. So if that fat got infected and inflamed, it might directly affect the heart.” That could help explain cardiovascular problems associated with Covid-19 infections.
Looking at tissue taken from autopsy, there was evidence of SARS-CoV-2 virus inside the fat cells as well as surrounding inflammation. In fat cells and immune cells harvested from health humans, infection in the laboratory drove "an inflammatory response, particularly in the macrophages…They secreted proteins that are typically seen in a cytokine storm” where the immune response runs amok with potential life-threatening consequences. This suggests to McLaughlin “that there could be a regional and even a systemic inflammatory response following infection in fat.”
It is easy to see how the airborne SARS-CoV-2 virus infects the nose and lungs, but how does it get into fat tissue? That is a mystery and the source of ample speculation.
The macrophages studied by McLaughlin and Blish were spewing out inflammatory proteins, While the the virus within them was replicating, the new viral particles were not able to replicate within those cells. It was a different story in the fat cells. “When [the virus] gets into the fat cells, it not only replicates, it's a productive infection, which means the resulting viral particles can infect another cell,” including microphages, McLaughlin explains. It seems to be a symbiotic tango of the virus between the two cell types that keeps the cycle going.
It is easy to see how the airborne SARS-CoV-2 virus infects the nose and lungs, but how does it get into fat tissue? That is a mystery and the source of ample speculation.
Macrophages are mobile; they engulf and carry invading pathogens to lymphoid tissue in the lymph nodes, tonsils and elsewhere in the body to alert T cells of the immune system to the pathogen. Perhaps some of them also carry the virus through the bloodstream to more distant tissue.
ACE2 receptors are the means by which SARS-CoV-2 latches on to and enters most cells. They are not thought to be common on fat cells, so initially most researchers thought it unlikely they would become infected.
However, while some cell receptors always sit on the surface of the cell, other receptors are expressed on the surface only under certain conditions. Philipp Scherer, a professor of internal medicine and director of the Touchstone Diabetes Center at the University of Texas Southwestern Medical Center, suggests that, in people who have obesity, “There might be higher levels of dysfunctional [fat cells] that facilitate entry of the virus,” either through transiently expressed ACE2 or other receptors. Inflammatory proteins generated by macrophages might contribute to this process.
Another hypothesis is that viral RNA might be smuggled into fat cells as cargo in small bits of material called extracellular vesicles, or EVs, that can travel between cells. Other researchers have shown that when EVs express ACE2 receptors, they can act as decoys for SARS-CoV-2, where the virus binds to them rather than a cell. These scientists are working to create drugs that mimic this decoy effect as an approach to therapy.
Do fat cells play a role in Long Covid? “Fat cells are a great place to hide. You have all the energy you need and fat cells turn over very slowly; they have a half-life of ten years,” says Scherer. Observational studies suggest that acute Covid-19 can trigger the onset of diabetes especially in people who are overweight, and that patients taking medicines to regulate their diabetes “were actually quite protective” against acute Covid-19. Scherer has funding to study the risks and benefits of those drugs in animal models of Long Covid.
McLaughlin says there are two areas of potential concern with fat tissue and Long Covid. One is that this tissue might serve as a “big reservoir where the virus continues to replicate and is sent out” to other parts of the body. The second is that inflammation due to infected fat cells and macrophages can result in fibrosis or scar tissue forming around organs, inhibiting their function. Once scar tissue forms, the tissue damage becomes more difficult to repair.
Current Covid-19 treatments work by stopping the virus from entering cells through the ACE2 receptor, so they likely would have no effect on virus that uses a different mechanism. That means another approach will have to be developed to complement the treatments we already have. So the best advice McLaughlin can offer today is to keep current on vaccinations and boosters and lose weight to reduce the risk associated with obesity.
Why we should put insects on the menu
Insects for sale at a market in Cambodia.
I walked through the Dong Makkhai forest-products market, just outside of Vientiane, the laid-back capital of the Lao Peoples Democratic Republic or Lao PDR. Piled on rough display tables were varieties of six-legged wildlife–grasshoppers, small white crickets, house crickets, mole crickets, wasps, wasp eggs and larvae, dragonflies, and dung beetles. Some were roasted or fried, but in a few cases, still alive and scrabbling at the bottom of deep plastic bowls. I crunched on some fried crickets and larvae.
One stall offered Giant Asian hornets, both babies and adults. I suppressed my inner squirm and, in the interests of world food security and equity, accepted an offer of the soft, velvety larva; they were smooth on the tongue and of a pleasantly cool, buttery-custard consistency. Because the seller had already given me a free sample, I felt obliged to buy a chunk of the nest with larvae and some dead adults, which the seller mixed with kaffir lime leaves.
The year was 2016 and I was in Lao PDR because Veterinarians without Borders/Vétérinaires sans Frontières-Canada had initiated a project on small-scale cricket farming. The intent was to organize and encourage rural women to grow crickets as a source of supplementary protein and sell them at the market for cash. As a veterinary epidemiologist, I had been trained to exterminate disease spreading insects—Lyme disease-carrying ticks, kissing bugs that carry American Sleeping Sickness and mosquitoes carrying malaria, West Nile and Zika. Now, as part of a global wave promoting insects as a sustainable food source, I was being asked to view arthropods as micro-livestock, and devise management methods to keep them alive and healthy. It was a bit of a mind-bender.
The 21st century wave of entomophagy, or insect eating, first surged in the early 2010s, promoted by a research centre in Wageningen, a university in the Netherlands, conferences organized by the Food and Agriculture Organization of the United Nations, and enthusiastic endorsements by culinary adventurers and celebrities from Europeanized cultures. Headlines announced that two billion people around the world already ate insects, and that if everyone adopted entomophagy we could reduce greenhouse gases, mitigate climate change, and reign in profligate land and water use associated with industrial livestock production.
Furthermore, eating insects was better for human health than eating beef. If we were going to feed the estimated nine billion people with whom we will share the earth in 2050, we would need to make some radical changes in our agriculture and food systems. As one author proclaimed, entomophagy presented us with a last great chance to save the planet.
In 2010, in Kunming, a friend had served me deep-fried bamboo worms. I ate them to be polite. They tasted like French fries, but with heads.
The more recent data suggests that the number of people who eat insects in various forms, though sizeable, may be closer to several hundreds of millions. I knew that from several decades of international veterinary work. Sometimes, for me, insect eating has been simply a way of acknowledging cultural diversity. In 2010, in Kunming, a friend had served me deep-fried bamboo worms. I ate them to be polite. They tasted like French fries, but with heads. My friend said he preferred them chewier. I never thought about them much after that. I certainly had not thought about them as ingredients for human health.
Is consuming insects good for human health? Researchers over the past decade have begun to tease that apart. Some think it might not be useful to use the all-encompassing term insect at all; we don’t lump cows, pigs, chickens into one culinary category. Which insects are we talking about? What are they fed? Were they farmed or foraged? Which stages of the insects are we eating? Do we eat them directly or roasted and ground up?
The overall research indicates that, in general, the usual farmed insects (crickets, locusts, mealworms, soldier fly larvae) have high levels of protein and other important nutrients. If insects are foraged by small groups in Laos, they provide excellent food supplements. Large scale foraging in response to global markets can be incredibly destructive, but soldier fly larvae fed on food waste and used as a substitute for ground up anchovies for farmed fish (as Enterra Feed in Canada does) improves ecological sustainability.
Entomophagy alone might not save the planet, but it does give us an unprecedented opportunity to rethink how we produce and harvest protein.

The author enjoys insects from the Dong Makkhai forest-products market, just outside of Vientiane, the capital of the Lao Peoples Democratic Republic.
David Waltner-Toews
Between 1961 and 2018, world chicken production increased from 4 billion to 20 billion, pork from 200 million to over 100 billion pigs, human populations doubled from 3.5 billion to more than 7 billion, and life expectancy (on average) from 52 to 72 years. These dramatic increases in food production are the result of narrowly focused scientific studies, identifying specific nutrients, antibiotics, vaccines and genetics. What has been missing is any sort of peripheral vision: what are the unintended consequences of our narrowly defined success?
If we look more broadly, we can see that this narrowly defined success led to industrial farming, which caused wealth, health and labor inequities; polluted the environment; and created grounds for disease outbreaks. Recent generations of Europeanized people inherited the ideas of eating cows, pigs and chickens, along with their products, so we were focused only on growing them as efficiently as possible. With insects, we have an exciting chance to start from scratch. Because, for Europeanized people, insect eating is so strange, we are given the chance to reimagine our whole food system in consultation with local experts in Asia and Africa (many of them villagers), and to bring together the best of both locally adapted food production and global distribution.
For this to happen, we will need to change the dietary habits of the big meat eaters. How can we get accustomed to eating bugs? There’s no one answer, but there are a few ways. In many cases, insects are ground up and added as protein supplements to foods like crackers or bars. In certain restaurants, the chefs want you to get used to seeing the bugs as you eat them. At Le Feston Nu in Paris, the Arlo Guthrie look-alike bartender poured me a beer and brought out five small plates, each featuring a different insect in a nest of figs, sun-dried tomatoes, raisins, and chopped dried tropical fruits: buffalo worms, crickets, large grasshoppers (all just crunchy and no strong flavour, maybe a little nutty), small black ants (sour bite), and fat grubs with a beak, which I later identified as palm weevil larvae, tasting a bit like dried figs.
Some entomophagy advertising has used esthetically pleasing presentations in classy restaurants. In London, at the Archipelago restaurant, I dined on Summer Nights (pan fried chermoula crickets, quinoa, spinach and dried fruit), Love-Bug Salad (baby greens with an accompanying dish of zingy, crunchy mealworms fried in olive oil, chilis, lemon grass, and garlic), Bushman’s Cavi-Err (caramel mealworms, bilinis, coconut cream and vodka jelly), and Medieaval Hive (brown butter ice cream, honey and butter caramel sauce and a baby bee drone).

The Archipelago restaurant in London serves up a Love-Bug Salad: baby greens with an accompanying dish of zingy, crunchy mealworms fried in olive oil, chilis, lemon grass, and garlic.
David Waltner-Toews
Some chefs, like Tokyo-based Shoichi Uchiyama, try to entice people with sidewalk cooking lessons. Uchiyama's menu included hornet larvae, silkworm pupae, and silkworms. The silkworm pupae were white and pink and yellow. We snipped off the ends and the larvae dropped out. My friend Zen Kawabata roasted them in a small pan over a camp stove in the street to get the "chaff" off. We made tea from the feces of worms that had fed on cherry blossoms—the tea smelled of the blossoms. One of Uchiyama-san’s assistants made noodles from buckwheat dough that included powdered whole bees.
At a book reading in a Tokyo bookstore, someone handed me a copy of the Japanese celebrity scandal magazine Friday, opened to an article celebrating the “charms of insect eating.” In a photo, scantily-clad girls were drinking vodka and nibbling giant water bugs dubbed as toe-biters, along with pickled and fried locusts and butterfly larvae. If celebrities embraced bug-eating, others might follow. When asked to prepare an article on entomophagy for the high fashion Sorbet Magazine, I started by describing a clip of Nicole Kidman delicately snacking on insects.
Taking a page from the success story of MacDonald’s, we might consider targeting children and school lunches. Kids don’t lug around the same dietary baggage as the grownups, and they can carry forward new eating habits for the long term. When I offered roasted crickets to my grandchildren, they scarfed them down. I asked my five-year-old granddaughter what she thought: she preferred the mealworms to the crickets – they didn’t have legs that caught in her teeth.
Entomo Farms in Ontario, the province where I live, was described in 2015 by Canadian Business magazine as North America’s largest supplier of edible insects for human consumption. When visiting, I popped some of their roasted crickets into my mouth. They were crunchy, a little nutty. Nothing to get squeamish over. Perhaps the human consumption is indeed growing—my wife, at least, has joined me in my entomophagy adventures. When we celebrated our wedding anniversary at the Public Bar and Restaurant in Brisbane, Australia, the “Kang Kong Worms” and “Salmon, Manuka Honey, and Black Ants” seemed almost normal. Of course, the champagne helped.
In this episode of Making Sense of Science, my guest is Raina Plowright, a leading researcher when it comes to how and why viruses sometimes jump from bats to humans.
For this podcast episode, my guest is Raina Plowright, one of the world’s leading researchers when it comes to how and why viruses sometimes jump from bats to humans. The intuition may be that bats are the bad guys in this situation, but the real culprits are more likely humans and ways that we intrude on nature.
Plowright is a Cornell Atkinson Scholar and professor at Cornell in the Department of Public and Ecosystem Health in the College of Veterinary Medicine. Read her full bio here. For a shorter (and lightly edited) version of this conversation, you can check out my Q&A interview with Plowright in the single-issue magazine, One Health / One Planet, published earlier this month by Leaps.org in collaboration with the Aspen Institute and the Science Philanthropy Alliance.
In the episode, Plowright tells me about her global research team that is busy studying the complex chain of events in between viruses originating in bats and humans getting infected with those viruses. She’s collecting samples from bats in Asia, Africa and Australia, which sounds challenging enough, but now consider the diligence required to parse out 1400 different bat species.
We also discuss a high-profile paper that she co-authored last month arguing for greater investment in preventing pandemics in the first place instead of the current approach, which basically puts all of our eggs in the basket of trying to respond to these outbreaks after the fact. Investing in pandemic prevention is a small price to pay compared with millions of people killed and trillions of dollars spent during the response to COVID-19.
Listen to the Episode
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google

Raina Plowright, a disease ecologist at Cornell University, is taking blood and urine samples from hundreds of animals and using GPS tags to follow their movement.
Kelly Gorham

