The Skinny on Fat and Covid-19
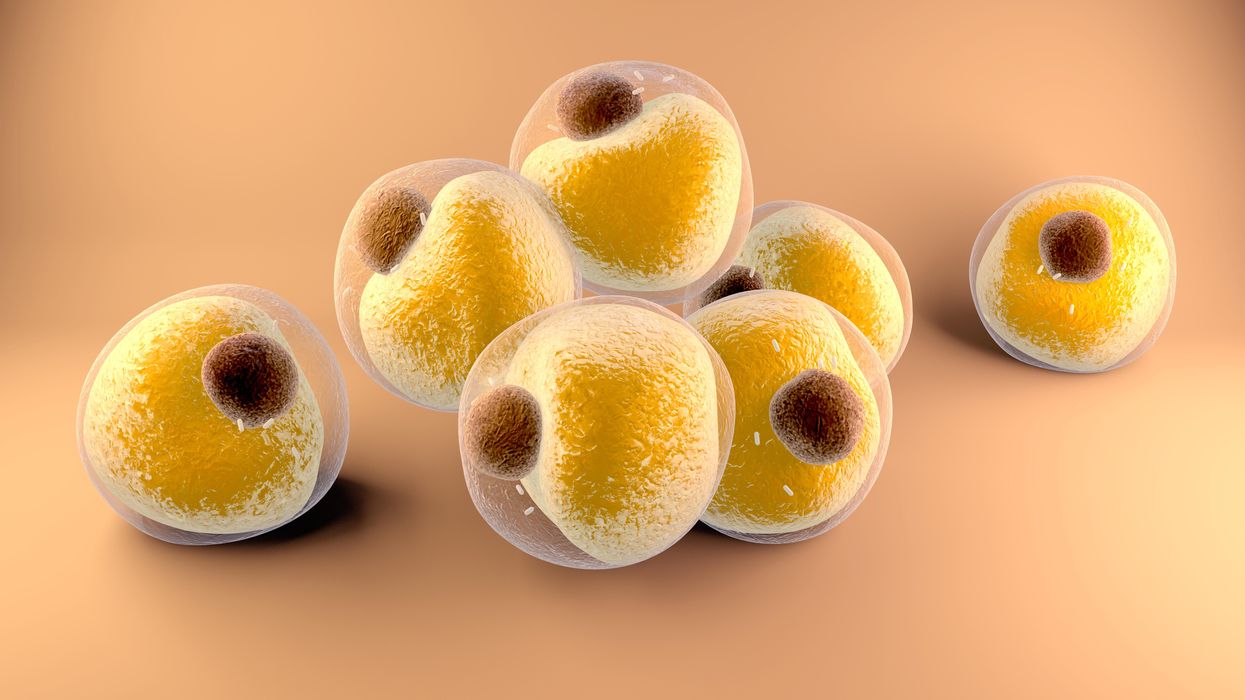
Researchers at Stanford have found that the virus that causes Covid-19 can infect fat cells, which could help explain why obesity is linked to worse outcomes for those who catch Covid-19.
Obesity is a risk factor for worse outcomes for a variety of medical conditions ranging from cancer to Covid-19. Most experts attribute it simply to underlying low-grade inflammation and added weight that make breathing more difficult.
Now researchers have found a more direct reason: SARS-CoV-2, the virus that causes Covid-19, can infect adipocytes, more commonly known as fat cells, and macrophages, immune cells that are part of the broader matrix of cells that support fat tissue. Stanford University researchers Catherine Blish and Tracey McLaughlin are senior authors of the study.
Most of us think of fat as the spare tire that can accumulate around the middle as we age, but fat also is present closer to most internal organs. McLaughlin's research has focused on epicardial fat, “which sits right on top of the heart with no physical barrier at all,” she says. So if that fat got infected and inflamed, it might directly affect the heart.” That could help explain cardiovascular problems associated with Covid-19 infections.
Looking at tissue taken from autopsy, there was evidence of SARS-CoV-2 virus inside the fat cells as well as surrounding inflammation. In fat cells and immune cells harvested from health humans, infection in the laboratory drove "an inflammatory response, particularly in the macrophages…They secreted proteins that are typically seen in a cytokine storm” where the immune response runs amok with potential life-threatening consequences. This suggests to McLaughlin “that there could be a regional and even a systemic inflammatory response following infection in fat.”
It is easy to see how the airborne SARS-CoV-2 virus infects the nose and lungs, but how does it get into fat tissue? That is a mystery and the source of ample speculation.
The macrophages studied by McLaughlin and Blish were spewing out inflammatory proteins, While the the virus within them was replicating, the new viral particles were not able to replicate within those cells. It was a different story in the fat cells. “When [the virus] gets into the fat cells, it not only replicates, it's a productive infection, which means the resulting viral particles can infect another cell,” including microphages, McLaughlin explains. It seems to be a symbiotic tango of the virus between the two cell types that keeps the cycle going.
It is easy to see how the airborne SARS-CoV-2 virus infects the nose and lungs, but how does it get into fat tissue? That is a mystery and the source of ample speculation.
Macrophages are mobile; they engulf and carry invading pathogens to lymphoid tissue in the lymph nodes, tonsils and elsewhere in the body to alert T cells of the immune system to the pathogen. Perhaps some of them also carry the virus through the bloodstream to more distant tissue.
ACE2 receptors are the means by which SARS-CoV-2 latches on to and enters most cells. They are not thought to be common on fat cells, so initially most researchers thought it unlikely they would become infected.
However, while some cell receptors always sit on the surface of the cell, other receptors are expressed on the surface only under certain conditions. Philipp Scherer, a professor of internal medicine and director of the Touchstone Diabetes Center at the University of Texas Southwestern Medical Center, suggests that, in people who have obesity, “There might be higher levels of dysfunctional [fat cells] that facilitate entry of the virus,” either through transiently expressed ACE2 or other receptors. Inflammatory proteins generated by macrophages might contribute to this process.
Another hypothesis is that viral RNA might be smuggled into fat cells as cargo in small bits of material called extracellular vesicles, or EVs, that can travel between cells. Other researchers have shown that when EVs express ACE2 receptors, they can act as decoys for SARS-CoV-2, where the virus binds to them rather than a cell. These scientists are working to create drugs that mimic this decoy effect as an approach to therapy.
Do fat cells play a role in Long Covid? “Fat cells are a great place to hide. You have all the energy you need and fat cells turn over very slowly; they have a half-life of ten years,” says Scherer. Observational studies suggest that acute Covid-19 can trigger the onset of diabetes especially in people who are overweight, and that patients taking medicines to regulate their diabetes “were actually quite protective” against acute Covid-19. Scherer has funding to study the risks and benefits of those drugs in animal models of Long Covid.
McLaughlin says there are two areas of potential concern with fat tissue and Long Covid. One is that this tissue might serve as a “big reservoir where the virus continues to replicate and is sent out” to other parts of the body. The second is that inflammation due to infected fat cells and macrophages can result in fibrosis or scar tissue forming around organs, inhibiting their function. Once scar tissue forms, the tissue damage becomes more difficult to repair.
Current Covid-19 treatments work by stopping the virus from entering cells through the ACE2 receptor, so they likely would have no effect on virus that uses a different mechanism. That means another approach will have to be developed to complement the treatments we already have. So the best advice McLaughlin can offer today is to keep current on vaccinations and boosters and lose weight to reduce the risk associated with obesity.
Podcast: New Solutions to Combat Gluten Sensitivities and Food Allergies
Biotech company Ukko is designing proteins that will be safe for everyone to eat, starting with peanut and gluten.
The "Making Sense of Science" podcast features interviews with leading medical and scientific experts about the latest developments and the big ethical and societal questions they raise. This monthly podcast is hosted by journalist Kira Peikoff, founding editor of the award-winning science outlet Leaps.org.
This month, we talk Anat Binur, the CEO of Israeli/U.S.-based biotech company Ukko. Ukko is taking a revolutionary approach to the distressing problem of food allergies and gluten sensitivities: their scientists are designing and engineering proteins that keep the good biophysical properties of the original proteins, while removing the immune-triggering parts that can cause life-threatening allergies. The end goal is proteins that are safe for everyone. Ukko is focusing first on developing a new safe gluten protein for use in baking and a new peanut protein for use as a therapeutic. Their unique platform could theoretically be used for any protein-based allergy, including cats and bees. Hear more in this episode.
Watch the 60-second trailer
Listen to the whole episode
<div id="buzzsprout-player-9950980"></div><script src="https://www.buzzsprout.com/1714953/9950980-solving-food-allergies-with-biotech-company-ukko.js?container_id=buzzsprout-player-9950980&player=small" type="text/javascript" charset="utf-8"></script>
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
Can a Non-Invasive Magnetic Helmet Treat Brain Cancer?
Glioblastoma is an aggressive and deadly brain cancer, causing more than 10,000 deaths in the US per year. In the last 30 years there has only been limited improvement in the survival rate despite advances in radiation therapy and chemotherapy. Today the typical survival rate is just 14 months and that extra time is spent suffering from the adverse and often brutal effects of radiation and chemotherapy.
Scientists are trying to design more effective treatments for glioblastoma with fewer side effects, and a team at the Department of Neurosurgery at Houston Methodist Hospital has created a magnetic helmet-based treatment called oncomagnetic therapy: a promising non-invasive treatment for shrinking cancerous tumors. In the first patient tried, the device was able to reduce the tumor of a glioblastoma patient by 31%. The researchers caution, however, that much more research is needed to determine its safety and effectiveness.
How It Works
“The whole idea originally came from a conversation I had with General Norman Schwarzkopf, a supposedly brilliant military strategist,” says David Baskin, professor of neurosurgery and leader of the effort at Houston Methodist. “I asked him what is the secret to your success and he said, ‘Energy. Take out the power grid and the enemy can't communicate.’ So I thought about what supplies [energy to] cancer, especially brain cancer.”
Baskin came up with the idea of targeting the mitochondria, which process and produce energy for cancer cells.
"This is the most exciting thing in glioblastoma treatment I've seen since I've been a neurosurgeon, but it is very preliminary,” Baskin says.
The magnetic helmet creates a powerful oscillating magnetic field. At a set range of frequencies and timings, it disrupts the flow of electrons in the mitochondria of cancer cells. This leads to a release of certain chemicals called Reactive Oxygen Species, or ROS. In normal cells, this excess ROS is much lower, and it's neutralized by other chemicals called antioxidants.
However, cancer cells already have more ROS: they grow rapidly and uncontrollably, so their mitochondria need to produce more energy which in turn generates more ROS. By using the powerful magnetic field, levels of ROS get so high that the malignant cells are torn apart.
The biggest challenge was working out the specific range of frequencies and timing parameters they needed to use to kill cancer cells. It took skill, intuition, luck and lots of experiments. The helmet could theoretically be used to treat all types of glioblastoma.
Developing the magnetic helmet was a collaborative process. Santosh Helekar is a neuroscientist at Houston Methodist Research Institute and the director of oncomagnetics (magnetic cancer therapies) at the Peak Center in Houston Methodist Hospital. His previous invention with colleagues gave the team a starting point to build on. “About 7 years back I developed a portable brain magnetic stimulation device to conduct brain research,” Helekar says. “We [then] conducted a pilot clinical trial in stroke patients. The results were promising.”
Helekar presented his findings to neurosurgeons including Baskin. They decided to collaborate. With a team of scientists behind them, they modified the device to kill cancer cells.

The magnetic helmet studied for treatment of glioblastoma
Dr. David Baskin
Initial Results
After success in the lab, the team got FDA approval to conduct a compassionate trial in a 53-year-old man with end-stage glioblastoma. He had tried every other treatment available. But within 30 days of using the magnetic helmet his tumor shrank by 31%.
Sadly, 36 days into the treatment, the patient had an unrelated head injury due to a fall. The treatment was paused and he later died of the injury. Autopsy results of his brain highlighted the dramatic reduction in tumor cells.
Baskin says, “This is the most exciting thing in glioblastoma treatment I've seen since I've been a neurosurgeon, but it is very preliminary.”
The helmet is part of a growing number of non-invasive cancer treatments. One device that is currently being used by glioblastoma patients is Optune. It uses electric fields called tumor treating fields to slow down cell division and has been through a successful phase 3 clinical trial.
The magnetic helmet has the promise to be another useful non-invasive treatment according to Professor Gabriel Zada, a neurosurgeon and director of the USC Brain Tumor Center. “We're learning that various electromagnetic fields and tumor treating fields appear to play a role in glioblastoma. So there is some precedent for this though the tumor treating fields work a little differently. I think there is major potential for it to be effective but of course it will require some trials.”
Professor Jonathan Sherman, a neurosurgeon and director of neuro-oncology at West Virginia University, reiterates the need for further testing. “It sounds interesting but it’s too early to tell what kind of long-term efficacy you get. We do not have enough data. Also if you’re disrupting [the magnetic field] you could negatively impact a patient. You could be affecting the normal conduction of electromagnetic activity in the brain.”
The team is currently extending their research. They are now testing the treatment in two other patients with end-stage glioblastoma. The immediate challenge is getting FDA approval for those at an earlier stage of the disease who are more likely to benefit.
The Future
Baskin and the team are designing a clinical trial in the U.S., .U.K. and Germany. After positive results in cell cultures, they’re in negotiations to collaborate with other researchers in using the technology for lung and breast cancer. With breast cancer, the soft tissue is easier to access so a magnetic device could be worn over the breast.
“My hope is to develop a treatment to treat and hopefully cure glioblastoma without radiation or chemotherapy,” Baskin says. “We're onto a strategy that could make a huge difference for patients with this disease and probably for patients with many other forms of cancer.”