The Science of Why Adjusting to Omicron Is So Tough

A brain expert weighs in on the cognitive biases that hold us back from adjusting to the new reality of Omicron.
We are sticking our heads into the sand of reality on Omicron, and the results may be catastrophic.
Omicron is over 4 times more infectious than Delta. The Pfizer two-shot vaccine offers only 33% protection from infection. A Pfizer booster vaccine does raises protection to about 75%, but wanes to around 30-40 percent 10 weeks after the booster.
The only silver lining is that Omicron appears to cause a milder illness than Delta. Yet the World Health Organization has warned about the “mildness” narrative.
That’s because the much faster disease transmission and vaccine escape undercut the less severe overall nature of Omicron. That’s why hospitals have a large probability of being overwhelmed, as the Center for Disease Control warned, in this major Omicron wave.
Yet despite this very serious threat, we see the lack of real action. The federal government tightened international travel guidelines and is promoting boosters. Certainly, it’s crucial to get as many people to get their booster – and initial vaccine doses – as soon as possible. But the government is not taking the steps that would be the real game-changers.
Pfizer’s anti-viral drug Paxlovid decreases the risk of hospitalization and death from COVID by 89%. Due to this effectiveness, the FDA approved Pfizer ending the trial early, because it would be unethical to withhold the drug from people in the control group. Yet the FDA chose not to hasten the approval process along with the emergence of Omicron in late November, only getting around to emergency authorization in late December once Omicron took over. That delay meant the lack of Paxlovid for the height of the Omicron wave, since it takes many weeks to ramp up production, resulting in an unknown number of unnecessary deaths.
We humans are prone to falling for dangerous judgment errors called cognitive biases.
Widely available at-home testing would enable people to test themselves quickly, so that those with mild symptoms can quarantine instead of infecting others. Yet the federal government did not make tests available to patients when Omicron emerged in late November. That’s despite the obviousness of the coming wave based on the precedent of South Africa, UK, and Denmark and despite the fact that the government made vaccines freely available. Its best effort was to mandate that insurance cover reimbursements for these kits, which is way too much of a barrier for most people. By the time Omicron took over, the federal government recognized its mistake and ordered 500 million tests to be made available in January. However, that’s far too late. And the FDA also played a harmful role here, with its excessive focus on accuracy going back to mid-2020, blocking the widespread availability of cheap at-home tests. By contrast, Europe has a much better supply of tests, due to its approval of quick and slightly less accurate tests.
Neither do we see meaningful leadership at the level of employers. Some are bringing out the tired old “delay the office reopening” play. For example, Google, Uber, and Ford, along with many others, have delayed the return to the office for several months. Those that already returned are calling for stricter pandemic measures, such as more masks and social distancing, but not changing their work arrangements or adding sufficient ventilation to address the spread of COVID.
Despite plenty of warnings from risk management and cognitive bias experts, leaders are repeating the same mistakes we fell into with Delta. And so are regular people. For example, surveys show that Omicron has had very little impact on the willingness of unvaccinated Americans to get a first vaccine dose, or of vaccinated Americans to get a booster. That’s despite Omicron having taken over from Delta in late December.
What explains this puzzling behavior on both the individual and society level? We humans are prone to falling for dangerous judgment errors called cognitive biases. Rooted in wishful thinking and gut reactions, these mental blindspots lead to poor strategic and financial decisions when evaluating choices.
These cognitive biases stem from the more primitive, emotional, and intuitive part of our brains that ensured survival in our ancestral environment. This quick, automatic reaction of our emotions represents the autopilot system of thinking, one of the two systems of thinking in our brains. It makes good decisions most of the time but also regularly makes certain systematic thinking errors, since it’s optimized to help us survive. In modern society, our survival is much less at risk, and our gut is more likely to compel us to focus on the wrong information to make decisions.
One of the biggest challenges relevant to Omicron is the cognitive bias known as the ostrich effect. Named after the myth that ostriches stick their heads into the sand when they fear danger, the ostrich effect refers to people denying negative reality. Delta illustrated the high likelihood of additional dangerous variants, yet we failed to pay attention to and prepare for such a threat.
We want the future to be normal. We’re tired of the pandemic and just want to get back to pre-pandemic times. Thus, we greatly underestimate the probability and impact of major disruptors, like new COVID variants. That cognitive bias is called the normalcy bias.
When we learn one way of functioning in any area, we tend to stick to that way of functioning. You might have heard of this as the hammer-nail syndrome: when you have a hammer, everything looks like a nail. That syndrome is called functional fixedness. This cognitive bias causes those used to their old ways of action to reject any alternatives, including to prepare for a new variant.
Our minds naturally prioritize the present. We want what we want now, and downplay the long-term consequences of our current desires. That fallacious mental pattern is called hyperbolic discounting, where we excessively discount the benefits of orienting toward the future and focus on the present. A clear example is focusing on the short-term perceived gains of trying to return to normal over managing the risks of future variants.
The way forward into the future is to defeat cognitive biases and avoid denying reality by rethinking our approach to the future.
The FDA requires a serious overhaul. It’s designed for a non-pandemic environment, where the goal is to have a highly conservative, slow-going, and risk-averse approach so that the public feels confident trusting whatever it approved. That’s simply unacceptable in a fast-moving pandemic, and we are bound to face future pandemics in the future.
The federal government needs to have cognitive bias experts weigh in on federal policy. Putting all of its eggs in one basket – vaccinations – is not a wise move when we face the risks of a vaccine-escaping variant. Its focus should also be on expediting and prioritizing anti-virals, scaling up cheap rapid testing, and subsidizing high-filtration masks.
For employers, instead of dictating a top-down approach to how employees collaborate, companies need to adopt a decentralized team-led approach. Each individual team leader of a rank-and-file employee team should determine what works best for their team. After all, team leaders tend to know much more of what their teams need, after all. Moreover, they can respond to local emergencies like COVID surges.
At the same time, team leaders need to be trained to integrate best practices for hybrid and remote team leadership. Companies transitioned to telework abruptly as part of the March 2020 lockdowns. They fell into the cognitive bias of functional fixedness and transposed their pre-existing, in-office methods of collaboration on remote work. Zoom happy hours are a clear example: The large majority of employees dislike them, and research shows they are disconnecting, rather than connecting.
Yet supervisors continue to use them, despite the existence of much better methods of facilitating colalboration, which have been shown to work, such as virtual water cooler discussions, virtual coworking, and virtual mentoring. Leaders also need to facilitate innovation in hybrid and remote teams through techniques such as virtual asynchronous brainstorming. Finally, team leaders need to adjust performance evaluation to adapt to the needs of hybrid and remote teams.
On an individual level, people built up certain expectations during the first two years of the pandemic, and they don't apply with Omicron. For example, most people still think that a cloth mask is a fine source of protection. In reality, you really need an N-95 mask, since Omicron is so much more infectious. Another example is that many people don’t realize that symptom onset is much quicker with Omicron, and they aren’t prepared for the consequences.
Remember that we have a huge number of people who are asymptomatic, often without knowing it, due to the much higher mildness of Omicron. About 8% of people admitted to hospitals for other reasons in San Francisco test positive for COVID without symptoms, which we can assume translates for other cities. That means many may think they're fine and they're actually infectious. The result is a much higher chance of someone getting many other people sick.
During this time of record-breaking cases, you need to be mindful about your internalized assumptions and adjust your risk calculus accordingly. So if you can delay higher-risk activities, January and February might be the time to do it. Prepare for waves of disruptions to continue over time, at least through the end of February.
Of course, you might also choose to not worry about getting infected. If you are vaccinated and boosted, and do not have any additional health risks, you are very unlikely to have a serious illness due to Omicron. You can just take the small risk of a serious illness – which can happen – and go about your daily life. If doing so, watch out for those you care about who do have health concerns, since if you infect them, they might not have a mild case even with Omicron.
In short, instead of trying to turn back the clock to the lost world of January 2020, consider how we might create a competitive advantage in our new future. COVID will never go away: we need to learn to live with it. That means reacting appropriately and thoughtfully to new variants and being intentional about our trade-offs.
New tech for prison reform spreads to 11 states
The U.S. has the highest incarceration rate in the world, costing $182 billion per year, partly because its antiquated data systems often fail to identify people who should be released. A tech nonprofit is trying to change that.
A new non-profit called Recidiviz is using data technology to reduce the size of the U.S. criminal justice system. The bi-coastal company (SF and NYC) is currently working with 11 states to improve their systems and, so far, has helped remove nearly 69,000 people — ones left floundering in jail or on parole when they should have been released.
“The root cause is fragmentation,” says Clementine Jacoby, 31, a software engineer who worked at Google before co-founding Recidiviz in 2019. In the 1970s and 80s, the U.S. built a series of disconnected data systems, and this patchwork is still being used by criminal justice authorities today. It requires parole officers to manually calculate release dates, leading to errors in many cases. “[They] have done everything they need to do to earn their release, but they're still stuck in the system,” Jacoby says.
Recidiviz has built a platform that connects the different databases, with the goal of identifying people who are already qualified for release but remain behind bars or on supervision. “Think of Recidiviz like Google Maps,” says Jacoby, who worked on Maps when she was at the tech giant. Google Maps takes in data from different sources – satellite images, street maps, local business data — and organizes it into one easy view. “Recidiviz does something similar with criminal justice data,” Jacoby explains, “making it easy to identify people eligible to come home or to move to less intensive levels of supervision.”
People like Jacoby’s uncle. His experience with incarceration is what inspired her passion for criminal justice reform in the first place.
The problems are vast
The U.S. has the highest incarceration rate in the world — 2 million people according to the watchdog group, Prison Policy Initiative — at a cost of $182 billion a year. The numbers could be a lot lower if not for an array of problems including inaccurate sentencing calculations, flawed algorithms and parole violations laws.
Sentencing miscalculations
To determine eligibility for release, the current system requires corrections officers to check 21 different requirements spread across five different databases for each of the 90 to 100 people under their supervision. These manual calculations are time prohibitive, says Jacoby, and fall victim to human error.
In addition, Recidiviz found that policies aimed at helping to reduce the prison population don’t always work correctly. A key example is time off for good behavior laws that allow inmates to earn one day off for every 30 days of good behavior. Some states' data systems are built to calculate time off as one day per month of good behavior, rather than per day. Over the course of a decade-long sentence, Jacoby says these miscalculations can lead to a huge discrepancy in the calculated release data and the actual release date.
Algorithms
Commercial algorithm-based software systems for risk assessment continue to be widely used in the criminal justice system, even though a 2018 study published in Science Advances exposed their limitations. After the study went viral, it took three years for the Justice Department to issue a report on their own flawed algorithms used to reduce the federal prison population as part of the 2018 First Step Act. The program, it was determined, overestimated the risk of putting inmates of color into early-release programs.
Despite its name, Recidiviz does not build these types of algorithms for predicting recidivism, or whether someone will commit another crime after being released from prison. Rather, Jacoby says the company’s "descriptive analytics” approach is specifically intended to weed out incarceration inequalities and avoid algorithmic pitfalls.
Parole violation laws
Research shows that 350,000 people a year — about a quarter of the total prison population — are sent back not because they’ve committed another crime, but because they’ve broken a specific rule of their probation. “Things that wouldn't send you or I to prison, but would send someone on parole,” such as crossing county lines or being in the presence of alcohol when they shouldn’t be, are inflating the prison population, says Jacoby.
It’s personal for the co-founder and CEO
“I grew up with an uncle who went into the prison system,” Jacoby says. At 19, he was sentenced to ten years in prison for a non-violent crime. A few months after being released from jail, he was sent back for a non-violent parole violation.
“For my family, the fact that one in four prison admissions are driven not by a crime but by someone who's broken a rule on probation and parole was really profound because that happened to my uncle,” Jacoby says. The experience led her to begin studying criminal justice in high school, then college. She continued her dive into how the criminal justice system works as part of her Passion Project while at Google, a program that allows employees to spend 20 percent of their time on pro-bono work. Two colleagues whose family members had also been stuck in the system joined her.
As part of the project, Jacoby interviewed hundreds of people involved in the criminal justice system. “Those on the right, those on the left, agreed that bad data was slowing down reform,” she says. Their research brought them to North Dakota where they began to understand the root of the problem. The corrections department is making “huge, consequential decisions every day [without] … the data,” Jacoby says. In a new video by Recidiviz not yet released, Jacoby recounts her exchange with the state’s director of corrections who told her, “‘It’s not that we have the data and we just don’t know how to make it public; we don’t have the information you think we have.'"
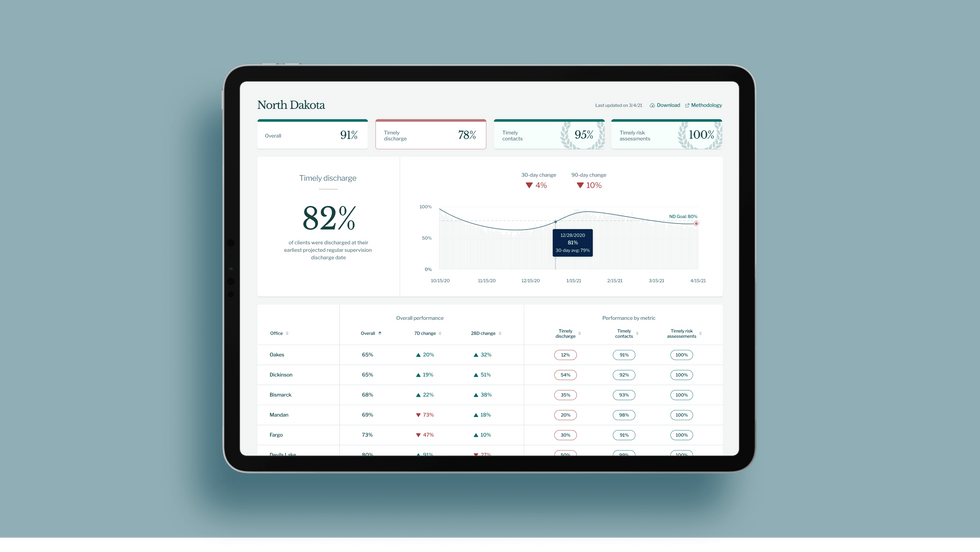
A mock-up (with fake data) of the types of dashboards and insights that Recidiviz provides to state governments.
Recidiviz
As a software engineer, Jacoby says the comment made no sense to her — until she witnessed it first-hand. “We spent a lot of time driving around in cars with corrections directors and parole officers watching them use these incredibly taxing, frankly terrible, old data systems,” Jacoby says.
As they weeded through thousands of files — some computerized, some on paper — they unearthed the consequences of bad data: Hundreds of people in prison well past their release date and thousands more whose release from parole was delayed because of minor paperwork issues. They found individuals stuck in parole because they hadn’t checked one last item off their eligibility list — like simply failing to provide their parole officer with a paystub. And, even when parolees advocated for themselves, the archaic system made it difficult for their parole officers to confirm their eligibility, so they remained in the system. Jacoby and her team also unpacked specific policies that drive racial disparities — such as fines and fees.
The Solution
It’s more than a trivial technical challenge to bring the incomplete, fragmented data onto a 21st century data platform. It takes months for Recidiviz to sift through a state’s information systems to connect databases “with the goal of tracking a person all the way through their journey and find out what’s working for 18- to 25-year-old men, what’s working for new mothers,” explains Jacoby in the video.
TED Talk: How bad data traps people in the U.S. justice system

TED Fellow Clementine Jacoby's TED Talk went live on Jan. 13. It describes how we can fix bad data in the criminal justice system, "bringing thousands of people home, reducing costs and improving public safety along the way."
Clementine Jacoby • TED2022
Ojmarrh Mitchell, an associate professor in the School of Criminology and Criminal Justice at Arizona State University, who is not involved with the company, says what Recidiviz is doing is “remarkable.” His perspective goes beyond academic analysis. In his pre-academic years, Mitchell was a probation officer, working within the framework of the “well known, but invisible” information sharing issues that plague criminal justice departments. The flexibility of Recidiviz’s approach is what makes it especially innovative, he says. “They identify the specific gaps in each jurisdiction and tailor a solution for that jurisdiction.”
On the downside, the process used by Recidiviz is “a bit opaque,” Mitchell says, with few details available on how Recidiviz designs its tools and tracks outcomes. By sharing more information about how its actions lead to progress in a given jurisdiction, Recidiviz could help reformers in other places figure out which programs have the best potential to work well.
The eleven states in which Recidiviz is working include California, Colorado, Maine, Michigan, Missouri, Pennsylvania and Tennessee. And a pilot program launched last year in Idaho, if scaled nationally, with could reduce the number of people in the criminal justice system by a quarter of a million people, Jacoby says. As part of the pilot, rather than relying on manual calculations, Recidiviz is equipping leaders and the probation officers with actionable information with a few clicks of an app that Recidiviz built.
Mitchell is disappointed that there’s even the need for Recidiviz. “This is a problem that government agencies have a responsibility to address,” he says. “But they haven’t.” For one company to come along and fill such a large gap is “remarkable.”
How Leqembi became the biggest news in Alzheimer’s disease in 40 years, and what comes next
Betsy Groves, 73, with her granddaughter. Groves learned in 2021 that she has Alzheimer's disease. She hopes to take Leqembi, a drug approved by the FDA last week.
A few months ago, Betsy Groves traveled less than a mile from her home in Cambridge, Mass. to give a talk to a bunch of scientists. The scientists, who worked for the pharmaceutical companies Biogen and Eisai, wanted to know how she lived her life, how she thought about her future, and what it was like when a doctor’s appointment in 2021 gave her the worst possible news. Groves, 73, has Alzheimer’s disease. She caught it early, through a lumbar puncture that showed evidence of amyloid, an Alzheimer’s hallmark, in her cerebrospinal fluid. As a way of dealing with her diagnosis, she joined the Alzheimer’s Association’s National Early-Stage Advisory Board, which helped her shift into seeing her diagnosis as something she could use to help others.
After her talk, Groves stayed for lunch with the scientists, who were eager to put a face to their work. Biogen and Eisai were about to release the first drug to successfully combat Alzheimer’s in 40 years of experimental disaster. Their drug, which is known by the scientific name lecanemab and the marketing name Leqembi, was granted accelerated approval by the U.S. Food and Drug Administration last Friday, Jan. 6, after a study in 1,800 people showed that it reduced cognitive decline by 27 percent over 18 months.
It is no exaggeration to say that this result is a huge deal. The field of Alzheimer’s drug development has been absolutely littered with failures. Almost everything researchers have tried has tanked in clinical trials. “Most of the things that we've done have proven not to be effective, and it's not because we haven’t been taking a ton of shots at goal,” says Anton Porsteinsson, director of the University of Rochester Alzheimer's Disease Care, Research, and Education Program, who worked on the lecanemab trial. “I think it's fair to say you don't survive in this field unless you're an eternal optimist.”
As far back as 1984, a cure looked like it was within reach: Scientists discovered that the sticky plaques that develop in the brains of those who have Alzheimer’s are made up of a protein fragment called beta-amyloid. Buildup of beta-amyloid seemed to be sufficient to disrupt communication between, and eventually kill, memory cells. If that was true, then the cure should be straightforward: Stop the buildup of beta-amyloid; stop the Alzheimer’s disease.
It wasn’t so simple. Over the next 38 years, hundreds of drugs designed either to interfere with the production of abnormal amyloid or to clear it from the brain flamed out in trials. It got so bad that neuroscience drug divisions at major pharmaceutical companies (AstraZeneca, Pfizer, Bristol-Myers, GSK, Amgen) closed one by one, leaving the field to smaller, scrappier companies, like Cambridge-based Biogen and Tokyo-based Eisai. Some scientists began to dismiss the amyloid hypothesis altogether: If this protein fragment was so important to the disease, why didn’t ridding the brain of it do anything for patients? There was another abnormal protein that showed up in the brains of Alzheimer’s patients, called tau. Some researchers defected to the tau camp, or came to believe the proteins caused damage in combination.
The situation came to a head in 2021, when the FDA granted provisional approval to a drug called aducanumab, marketed as Aduhelm, against the advice of its own advisory council. The approval was based on proof that Aduhelm reduced beta-amyloid in the brain, even though one research trial showed it had no effect on people’s symptoms or daily life. Aduhelm could also cause serious side effects, like brain swelling and amyloid related imaging abnormalities (known as ARIA, these are basically micro-bleeds that appear on MRI scans). Without a clear benefit to memory loss that would make these risks worth it, Medicare refused to pay for Aduhelm among the general population. Two congressional committees launched an investigation into the drug’s approval, citing corporate greed, lapses in protocol, and an unjustifiably high price. (Aduhelm was also produced by the pharmaceutical company Biogen.)
To be clear, Leqembi is not the cure Alzheimer’s researchers hope for. While the drug is the first to show clear signs of a clinical benefit, the scientific establishment is split on how much of a difference Leqembi will make in the real world.
So far, Leqembi is like Aduhelm in that it has been given accelerated approval only for its ability to remove amyloid from the brain. Both are monoclonal antibodies that direct the immune system to attack and clear dysfunctional beta-amyloid. The difference is that, while that’s all Aduhelm was ever shown to do, Leqembi’s makers have already asked the FDA to give it full approval – a decision that would increase the likelihood that Medicare will cover it – based on data that show it also improves Alzheimer’s sufferer’s lives. Leqembi targets a different type of amyloid, a soluble version called “protofibrils,” and that appears to change the effect. “It can give individuals and their families three, six months longer to be participating in daily life and living independently,” says Claire Sexton, PhD, senior director of scientific programs & outreach for the Alzheimer's Association. “These types of changes matter for individuals and for their families.”
To be clear, Leqembi is not the cure Alzheimer’s researchers hope for. It does not halt or reverse the disease, and people do not get better. While the drug is the first to show clear signs of a clinical benefit, the scientific establishment is split on how much of a difference Leqembi will make in the real world. It has “a rather small effect,” wrote NIH Alzheimer’s researcher Madhav Thambisetty, MD, PhD, in an email to Leaps.org. “It is unclear how meaningful this difference will be to patients, and it is unlikely that this level of difference will be obvious to a patient (or their caregivers).” Another issue is cost: Leqembi will become available to patients later this month, but Eisai is setting the price at $26,500 per year, meaning that very few patients will be able to afford it unless Medicare chooses to reimburse them for it.
The same side effects that plagued Aduhelm are common in Leqembi treatment as well. In many patients, amyloid doesn’t just accumulate around neurons, it also forms deposits in the walls of blood vessels. Blood vessels that are shot through with amyloid are more brittle. If you infuse a drug that targets amyloid, brittle blood vessels in the brain can develop leakage that results in swelling or bleeds. Most of these come with no symptoms, and are only seen during testing, which is why they are called “imaging abnormalities.” But in situations where patients have multiple diseases or are prescribed incompatible drugs, they can be serious enough to cause death. The three deaths reported from Leqembi treatment (so far) are enough to make Thambisetty wonder “how well the drug may be tolerated in real world clinical practice where patients are likely to be sicker and have multiple other medical conditions in contrast to carefully selected patients in clinical trials.”
Porsteinsson believes that earlier detection of Alzheimer’s disease will be the next great advance in treatment, a more important step forward than Leqembi’s approval.
Still, there are reasons to be excited. A successful Alzheimer’s drug can pave the way for combination studies, in which patients try a known effective drug alongside newer, more experimental ones; or preventative studies, which take place years before symptoms occur. It also represents enormous strides in researchers’ understanding of the disease. For example, drug dosages have increased massively—in some cases quadrupling—from the early days of Alzheimer’s research. And patient selection for studies has changed drastically as well. Doctors now know that you’ve got to catch the disease early, through PET-scans or CSF tests for amyloid, if you want any chance of changing its course.
Porsteinsson believes that earlier detection of Alzheimer’s disease will be the next great advance in treatment, a more important step forward than Leqembi’s approval. His lab already uses blood tests for different types of amyloid, for different types of tau, and for measures of neuroinflammation, neural damage, and synaptic health, but commercially available versions from companies like C2N, Quest, and Fuji Rebio are likely to hit the market in the next couple of years. “[They are] going to transform the diagnosis of Alzheimer's disease,” Porsteinsson says. “If someone is experiencing memory problems, their physicians will be able to order a blood test that will tell us if this is the result of changes in your brain due to Alzheimer's disease. It will ultimately make it much easier to identify people at a very early stage of the disease, where they are most likely to benefit from treatment.”
Learn more about new blood tests to detect Alzheimer's
Early detection can help patients for more philosophical reasons as well. Betsy Groves credits finding her Alzheimer’s early with giving her the space to understand and process the changes that were happening to her before they got so bad that she couldn’t. She has been able to update her legal documents and, through her role on the Advisory Group, help the Alzheimer’s Association with developing its programs and support services for people in the early stages of the disease. She still drives, and because she and her husband love to travel, they are hoping to get out of grey, rainy Cambridge and off to Texas or Arizona this spring.
Because her Alzheimer’s disease involves amyloid deposits (a “substantial portion” do not, says Claire Sexton, which is an additional complication for research), and has not yet reached an advanced stage, Groves may be a good candidate to try Leqembi. She says she’d welcome the opportunity to take it. If she can get access, Groves hopes the drug will give her more days to be fully functioning with her husband, daughters, and three grandchildren. Mostly, she avoids thinking about what the latter stages of Alzheimer’s might be like, but she knows the time will come when it will be her reality. “So whatever lecanemab can do to extend my more productive ways of engaging with relationships in the world,” she says. “I'll take that in a minute.”

