After spaceflight record, NASA looks to protect astronauts on even longer trips

NASA astronaut Frank Rubio floats by the International Space Station’s “window to the world.” Yesterday, he returned from the longest single spaceflight by a U.S. astronaut on record - over one year. Exploring deep space will require even longer missions.
At T-minus six seconds, the main engines of the Atlantis Space Shuttle ignited, rattling its capsule “like a skyscraper in an earthquake,” according to astronaut Tom Jones, describing the 1988 launch. As the rocket lifted off and accelerated to three times the force of Earth's gravity, “It felt as if two of my friends were standing on my chest and wouldn’t get off.” But when Atlantis reached orbit, the main engines cut off, and the astronauts were suddenly weightless.
Since 1961, NASA has sent hundreds of astronauts into space while working to making their voyages safer and smoother. Yet, challenges remain. Weightlessness may look amusing when watched from Earth, but it has myriad effects on cognition, movement and other functions. When missions to space stretch to six months or longer, microgravity can impact astronauts’ health and performance, making it more difficult to operate their spacecraft.
Yesterday, NASA astronaut Frank Rubio returned to Earth after over one year, the longest single spaceflight for a U.S. astronaut. But this is just the start; longer and more complex missions into deep space loom ahead, from returning to the moon in 2025 to eventually sending humans to Mars. To ensure that these missions succeed, NASA is increasing efforts to study the biological effects and prevent harm.
The dangers of microgravity are real
A NASA report published in 2016 details a long list of incidents and near-misses caused – at least partly – by space-induced changes in astronauts’ vision and coordination. These issues make it harder to move with precision and to judge distance and velocity.
According to the report, in 1997, a resupply ship collided with the Mir space station, possibly because a crew member bumped into the commander during the final docking maneuver. This mishap caused significant damage to the space station.
Returns to Earth suffered from problems, too. The same report notes that touchdown speeds during the first 100 space shuttle landings were “outside acceptable limits. The fastest landing on record – 224 knots (258 miles) per hour – was linked to the commander’s momentary spatial disorientation.” Earlier, each of the six Apollo crews that landed on the moon had difficulty recognizing moon landmarks and estimating distances. For example, Apollo 15 landed in an unplanned area, ultimately straddling the rim of a five-foot deep crater on the moon, harming one of its engines.
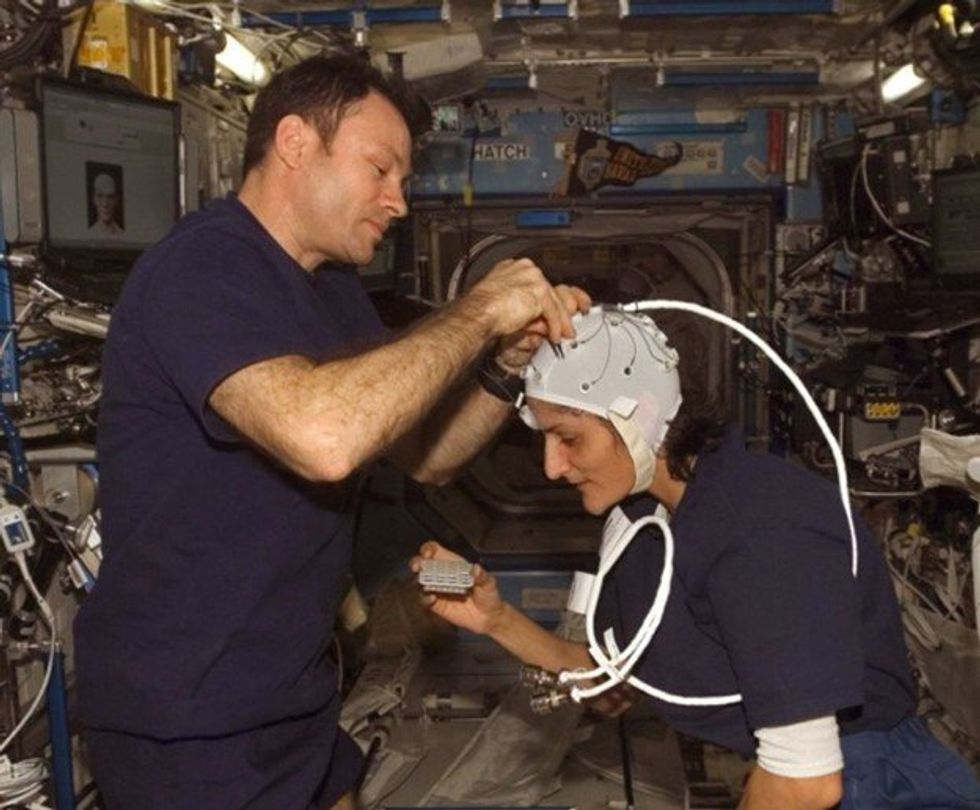
Spaceflight causes unique stresses on astronauts’ brains and central nervous systems. NASA is working to reduce these harmful effects.
NASA
Space messes up your brain
In space, astronauts face the challenges of microgravity, ionizing radiation, social isolation, high workloads, altered circadian rhythms, monotony, confined living quarters and a high-risk environment. Among these issues, microgravity is one of the most consequential in terms of physiological changes. It changes the brain’s structure and its functioning, which can hurt astronauts’ performance.
The brain shifts upwards within the skull, displacing the cerebrospinal fluid, which reduces the brain’s cushioning. Essentially, the brain becomes crowded inside the skull like a pair of too-tight shoes.
That’s partly because of how being in space alters blood flow. On Earth, gravity pulls our blood and other internal fluids toward our feet, but our circulatory valves ensure that the fluids are evenly distributed throughout the body. In space, there’s not enough gravity to pull the fluids down, and they shift up, says Rachael D. Seidler, a physiologist specializing in spaceflight at the University of Florida and principal investigator on many space-related studies. The head swells and legs appear thinner, causing what astronauts call “puffy face chicken legs.”
“The brain changes at the structural and functional level,” says Steven Jillings, equilibrium and aerospace researcher at the University of Antwerp in Belgium. “The brain shifts upwards within the skull,” displacing the cerebrospinal fluid, which reduces the brain’s cushioning. Essentially, the brain becomes crowded inside the skull like a pair of too-tight shoes. Some of the displaced cerebrospinal fluid goes into cavities within the brain, called ventricles, enlarging them. “The remaining fluids pool near the chest and heart,” explains Jillings. After 12 consecutive months in space, one astronaut had a ventricle that was 25 percent larger than before the mission.
Some changes reverse themselves while others persist for a while. An example of a longer-lasting problem is spaceflight-induced neuro-ocular syndrome, which results in near-sightedness and pressure inside the skull. A study of approximately 300 astronauts shows near-sightedness affects about 60 percent of astronauts after long missions on the International Space Station (ISS) and more than 25 percent after spaceflights of only a few weeks.
Another long-term change could be the decreased ability of cerebrospinal fluid to clear waste products from the brain, Seidler says. That’s because compressing the brain also compresses its waste-removing glymphatic pathways, resulting in inflammation, vulnerability to injuries and worsening its overall health.
The effects of long space missions were best demonstrated on astronaut twins Scott and Mark Kelly. This NASA Twins Study showed multiple, perhaps permanent, changes in Scott after his 340-day mission aboard the ISS, compared to Mark, who remained on Earth. The differences included declines in Scott’s speed, accuracy and cognitive abilities that persisted longer than six months after returning to Earth in March 2016.
By the end of 2020, Scott’s cognitive abilities improved, but structural and physiological changes to his eyes still remained, he said in a BBC interview.
“It seems clear that the upward shift of the brain and compression of the surrounding tissues with ventricular expansion might not be a good thing,” Seidler says. “But, at this point, the long-term consequences to brain health and human performance are not really known.”

NASA astronaut Kate Rubins conducts a session for the Neuromapping investigation.
NASA
Staying sharp in space
To investigate how prolonged space travel affects the brain, NASA launched a new initiative called the Complement of Integrated Protocols for Human Exploration Research (CIPHER). “CIPHER investigates how long-duration spaceflight affects both brain structure and function,” says neurobehavioral scientist Mathias Basner at the University of Pennsylvania, a principal investigator for several NASA studies. “Through it, we can find out how the brain adapts to the spaceflight environment and how certain brain regions (behave) differently after – relative to before – the mission.”
To do this, he says, “Astronauts will perform NASA’s cognition test battery before, during and after six- to 12-month missions, and will also perform the same test battery in an MRI scanner before and after the mission. We have to make sure we better understand the functional consequences of spaceflight on the human brain before we can send humans safely to the moon and, especially, to Mars.”
As we go deeper into space, astronauts cognitive and physical functions will be even more important. “A trip to Mars will take about one year…and will introduce long communication delays,” Seidler says. “If you are on that mission and have a problem, it may take eight to 10 minutes for your message to reach mission control, and another eight to 10 minutes for the response to get back to you.” In an emergency situation, that may be too late for the response to matter.
“On a mission to Mars, astronauts will be exposed to stressors for unprecedented amounts of time,” Basner says. To counter them, NASA is considering the continuous use of artificial gravity during the journey, and Seidler is studying whether artificial gravity can reduce the harmful effects of microgravity. Some scientists are looking at precision brain stimulation as a way to improve memory and reduce anxiety due to prolonged exposure to radiation in space.
Other scientists are exploring how to protect neural stem cells (which create brain cells) from radiation damage, developing drugs to repair damaged brain cells and protect cells from radiation.
To boldly go where no astronauts have gone before, they must have optimal reflexes, vision and decision-making. In the era of deep space exploration, the brain—without a doubt—is the final frontier.
Additionally, NASA is scrutinizing each aspect of the mission, including astronaut exercise, nutrition and intellectual engagement. “We need to give astronauts meaningful work. We need to stimulate their sensory, cognitive and other systems appropriately,” Basner says, especially given their extreme confinement and isolation. The scientific experiments performed on the ISS – like studying how microgravity affects the ability of tissue to regenerate is a good example.
“We need to keep them engaged socially, too,” he continues. The ISS crew, for example, regularly broadcasts from space and answers prerecorded questions from students on Earth, and can engage with social media in real time. And, despite tight quarters, NASA is ensuring the crew capsule and living quarters on the moon or Mars include private space, which is critical for good mental health.
Exploring deep space builds on a foundation that began when astronauts first left the planet. With each mission, scientists learn more about spaceflight effects on astronauts’ bodies. NASA will be using these lessons to succeed with its plans to build science stations on the moon and, eventually, Mars.
“Through internally and externally led research, investigations implemented in space and in spaceflight simulations on Earth, we are striving to reduce the likelihood and potential impacts of neurostructural changes in future, extended spaceflight,” summarizes NASA scientist Alexandra Whitmire. To boldly go where no astronauts have gone before, they must have optimal reflexes, vision and decision-making. In the era of deep space exploration, the brain—without a doubt—is the final frontier.
As gene therapies and small molecule drugs are being studied in clinical trials, companies increasingly see the value in hiring patients to help explain the potential benefits.
As a child, Wendy Borsari participated in a health study at Boston Children’s Hospital. She was involved because heart disease and sudden cardiac arrest ran in her family as far back as seven generations. When she was 18, however, the study’s doctors told her that she had a perfectly healthy heart and didn’t have to worry.
A couple of years after graduating from college, though, the Boston native began to experience episodes of near fainting. During any sort of strenuous exercise, my blood pressure would drop instead of increasing, she recalls.
She was diagnosed at 24 with hypertrophic cardiomyopathy. Although HCM is a commonly inherited heart disease, Borsari’s case resulted from a rare gene mutation, the MYH7 gene. Her mother had been diagnosed at 27, and Borsari had already lost her grandmother and two maternal uncles to the condition. After her own diagnosis, Borsari spent most of her free time researching the disease and “figuring out how to have this condition and still be the person I wanted to be,” she says.
Then, her son was found to have the genetic mutation at birth and diagnosed with HCM at 15. Her daughter, also diagnosed at birth, later suffered five cardiac arrests.
That changed Borsari’s perspective. She decided to become a patient advocate. “I didn’t want to just be a patient with the condition,” she says. “I wanted to be more involved with the science and the biopharmaceutical industry so I could be active in helping to make it better for other patients.”
She consulted on patient advocacy for a pharmaceutical and two foundations before coming to a company called Tenaya in 2021.
“One of our core values as a company is putting patients first,” says Tenaya's CEO, Faraz Ali. “We thought of no better way to put our money where our mouth is than by bringing in somebody who is affected and whose family is affected by a genetic form of cardiomyopathy to have them make sure we’re incorporating the voice of the patient.”
Biomedical corporations and government research agencies are now incorporating patient advocacy more than ever, says Alice Lara, president and CEO of the Sudden Arrhythmia Death Syndromes Foundation in Salt Lake City, Utah. These organizations have seen the effectiveness of including patient voices to communicate and exemplify the benefits that key academic research institutions have shown in their medical studies.
“From our side of the aisle,” Lara says, “what we know as patient advocacy organizations is that educated patients do a lot better. They have a better course in their therapy and their condition, and understanding the genetics is important because all of our conditions are genetic.”
Founded in 2016, Tenaya is advancing gene therapies and small molecule drugs in clinical trials for both prevalent and rare forms of heart disease, says Ali, the CEO.
The firm's first small molecule, now in a Phase 1 clinical trial, is intended to treat heart failure with preserved ejection fraction, where the amount of blood pumped by the heart is reduced due to the heart chambers becoming weak or stiff. The condition accounts for half or more of all heart failure in the U.S., according to Ali, and is growing quickly because it's closely associated with diabetes. It’s also linked with metabolic syndrome, or a cluster of conditions including high blood pressure, high blood sugar, excess body fat around the waist, and abnormal cholesterol levels.
“We have a novel molecule that is first in class and, to our knowledge, best in class to tackle that, so we’re very excited about the clinical trial,” Ali says.
The first phase of the trial is being performed with healthy participants, rather than people with the disease, to establish safety and tolerability. The researchers can also look for the drug in blood samples, which could tell them whether it's reaching its target. Ali estimates that, if the company can establish safety and that it engages the right parts of the body, it will likely begin dosing patients with the disease in 2024.
Tenaya’s therapy delivers a healthy copy of the gene so that it makes a copy of the protein missing from the patients' hearts because of their mutation. The study will start with adult patients, then pivot potentially to children and even newborns, Ali says, “where there is an even greater unmet need because the disease progresses so fast that they have no options.”
Although this work still has a long way to go, Ali is excited about the potential because the gene therapy achieved positive results in the preclinical mouse trial. This animal trial demonstrated that the treatment reduced enlarged hearts, reversed electrophysiological abnormalities, and improved the functioning of the heart by increasing the ejection fraction after the single-dose of gene therapy. That measurement remained stable to the end of the animals’ lives, roughly 18 months, Ali says.
He’s also energized by the fact that heart disease has “taken a page out of the oncology playbook” by leveraging genetic research to develop more precise and targeted drugs and gene therapies.
“Now we are talking about a potential cure of a disease for which there was no cure and using a very novel concept,” says Melind Desai of the Cleveland Clinic.
Tenaya’s second program focuses on developing a gene therapy to mitigate the leading cause of hypertrophic cardiomyopathy through a specific gene called MYPBC3. The disease affects approximately 600,000 patients in the U.S. This particular genetic form, Ali explains, affects about 115,000 in the U.S. alone, so it is considered a rare disease.
“There are infants who are dying within the first weeks to months of life as a result of this mutation,” he says. “There are also adults who start having symptoms in their 20s, 30s and 40s with early morbidity and mortality.” Tenaya plans to apply before the end of this year to get the FDA’s approval to administer an investigational drug for this disease humans. If approved, the company will begin to dose patients in 2023.
“We now understand the genetics of the heart much better,” he says. “We now understand the leading genetic causes of hypertrophic myopathy, dilated cardiomyopathy and others, so that gives us the ability to take these large populations and stratify them rationally into subpopulations.”
Melind Desai, MD, who directs Cleveland Clinic’s Hypertrophic Cardiomyopathy Center, says that the goal of Tenaya’s second clinical study is to help improve the basic cardiac structure in patients with hypertrophic cardiomyopathy related to the MYPBC3 mutation.
“Now we are talking about a potential cure of a disease for which there was no cure and using a very novel concept,” he says. “So this is an exciting new frontier of therapeutic investigation for MYPBC3 gene-positive patients with a chance for a cure.
Neither of Tenaya’s two therapies address the gene mutation that has affected Borsari and her family. But Ali sees opportunity down the road to develop a gene therapy for her particular gene mutation, since it is the second leading cause of cardiomyopathy. Treating the MYH7 gene is especially challenging because it requires gene editing or silencing, instead of just replacing the gene.

Wendy Borsari was diagnosed at age 24 with a commonly inherited heart disease. She joined Tenaya as a patient advocate in 2021.
Wendy Borsari
“If you add a healthy gene it will produce healthy copies,” Ali explains, “but it won’t stop the bad effects of the mutant protein the gene produces. You can only do that by silencing the gene or editing it out, which is a different, more complicated approach.”
Euan Ashley, professor of medicine and genetics at Stanford University and founding director of its Center for Inherited Cardiovascular Disease, is confident that we will see genetic therapies for heart disease within the next decade.
“We are at this really exciting moment in time where we have diseases that have been under-recognized and undervalued now being attacked by multiple companies with really modern tools,” says Ashley, author of The Genome Odyssey. “Gene therapies are unusual in the sense that they can reverse the cause of the disease, so we have the enticing possibility of actually reversing or maybe even curing these diseases.”
Although no one is doing extensive research into a gene therapy for her particular mutation yet, Borsari remains hopeful, knowing that companies such as Tenaya are moving in that direction.
“I know that’s now on the horizon,” she says. “It’s not just some pipe dream, but will happen hopefully in my lifetime or my kids’ lifetime to help them.”
Chicken that is grown entirely in a laboratory, without harming a single bird, could be sold in supermarkets in the coming months. But critics say the doubts about lab-grown meat have not been appropriately explored.
Last November, when the U.S. Food and Drug Administration disclosed that chicken from a California firm called UPSIDE Foods did not raise safety concerns, it drily upended how humans have obtained animal protein for thousands of generations.
“The FDA is ready to work with additional firms developing cultured animal cell food and production processes to ensure their food is safe and lawful,” the agency said in a statement at the time.
Assuming UPSIDE obtains clearances from the U.S. Department of Agriculture, its chicken – grown entirely in a laboratory without harming a single bird – could be sold in supermarkets in the coming months.
“Ultimately, we want our products to be available everywhere meat is sold, including retail and food service channels,” a company spokesperson said. The upscale French restaurant Atelier Crenn in San Francisco will have UPSIDE chicken on its menu once it is approved, she added.
Known as lab-grown or cultured meat, a product such as UPSIDE’s is created using stem cells and other tissue obtained from a chicken, cow or other livestock. Those cells are then multiplied in a nutrient-dense environment, usually in conjunction with a “scaffold” of plant-based materials or gelatin to give them a familiar form, such as a chicken breast or a ribeye steak. A Dutch company called Mosa Meat claims it can produce 80,000 hamburgers derived from a cluster of tissue the size of a sesame seed.
Critics say the doubts about lab-grown meat and the possibility it could merge “Brave New World” with “The Jungle” and “Soylent Green” have not been appropriately explored.
That’s a far cry from when it took months of work to create the first lab-grown hamburger a decade ago. That minuscule patty – which did not contain any fat and was literally plucked from a Petri dish to go into a frying pan – cost about $325,000 to produce.
Just a decade later, an Israeli company called Future Meat said it can produce lab-grown meat for about $1.70 per pound. It plans to open a production facility in the U.S. sometime in 2023 and distribute its products under the brand name “Believer.”
Costs for production have sunk so low that researchers at Carnegie Mellon University in Pittsburgh expect sometime in early 2024 to produce lab-grown Wagyu steak to showcase the viability of growing high-end cuts of beef cheaply. The Carnegie Mellon team is producing its Wagyu using a consumer 3-D printer bought secondhand on eBay and modified to print the highly marbled flesh using a method developed by the university. The device costs $200 – about the same as a pound of Wagyu in the U.S. The initiative’s modest five-figure budget was successfully crowdfunded last year.
“The big cost is going to be the cells (which are being extracted by a cow somewhere in Pennsylvania), but otherwise printing doesn’t add much to the process,” said Rosalyn Abbott, a Carnegie Mellon assistant professor of bioengineering who is co-leader on the project. “But it adds value, unlike doing this with ground meat.”
Lab-Grown Meat’s Promise
Proponents of lab-grown meat say it will cut down on traditional agriculture, which has been a leading contributor to deforestation, water shortages and contaminated waterways from animal waste, as well as climate change.
An Oxford University study from 2011 concludes lab-grown meat could have greenhouse emissions 96 percent lower compared to traditionally raised livestock. Moreover, proponents of lab-grown meat claim that the suffering of animals would decline dramatically, as they would no longer need to be warehoused and slaughtered. A recently opened 26-story high-rise in China dedicated to the raising and slaughtering of pigs illustrates the current plight of livestock in stark terms.
Scientists may even learn how to tweak lab-grown meat to make it more nutritious. Natural red meat is high in saturated fat and, if it’s eaten too often, can lead to chronic diseases. In lab versions, the saturated fat could be swapped for healthier, omega-3 fatty acids.
But critics say the doubts about lab-grown meat and the possibility it could merge “Brave New World” with “The Jungle” and “Soylent Green” have not been appropriately explored.
A Slippery Slope?
Some academics who have studied the moral and ethical issues surrounding lab-grown meat believe it will have a tough path ahead gaining acceptance by consumers. Should it actually succeed in gaining acceptance, many ethical questions must be answered.
“People might be interested” in lab-grown meat, perhaps as a curiosity, said Carlos Alvaro, an associate professor of philosophy at the New York City College of Technology, part of the City University of New York. But the allure of traditionally sourced meat has been baked – or perhaps grilled – into people’s minds for so long that they may not want to make the switch. Plant-based meat provides a recent example of the uphill battle involved in changing old food habits, with Beyond Meat’s stock prices dipping nearly 80 percent in 2022.
"There are many studies showing that people don’t really care about the environment (to that extent)," Alvaro said. "So I don’t know how you would convince people to do this because of the environment.”
“From my research, I understand that the taste (of lab-grown meat) is not quite there,” Alvaro said, noting that the amino acids, sugars and other nutrients required to grow cultivated meat do not mimic what livestock are fed. He also observed that the multiplication of cells as part of the process “really mimic cancer cells” in the way they grow, another off-putting thought for would-be consumers of the product.
Alvaro is also convinced the public will not buy into any argument that lab-grown meat is more environmentally friendly.
“If people care about the environment, they either try and consume considerably less meat and other animal products, or they go vegan or vegetarian,” he said. “But there are many studies showing that people don’t really care about the environment (to that extent). So I don’t know how you would convince people to do this because of the environment.”
Ben Bramble, a professor at Australian National University who previously held posts at Princeton and Trinity College in Ireland, takes a slightly different tack. He noted that “if lab-grown meat becomes cheaper, healthier, or tastier than regular meat, there will be a large market for it. If it becomes all of these things, it will dominate the market.”
However, Bramble has misgivings about that occurring. He believes a smooth transition from traditionally sourced meat to a lab-grown version would allow humans to elide over the decades of animal cruelty perpetrated by large-scale agriculture, without fully reckoning with and learning from this injustice.
“My fear is that if we all switch over to lab-grown meat because it has become cheaper, healthier, or tastier than regular meat, we might never come to realize what we have done, and the terrible things we are capable of,” he said. “This would be a catastrophe.”
Bramble’s writings about cultured meat also raise some serious moral conundrums. If, for example, animal meat may be cultivated without killing animals, why not create products from human protein?
Actually, that’s already happened.
It occurred in 2019, when Orkan Telhan, a professor of fine arts at the University of Pennsylvania, collaborated with two scientists to create an art exhibit at the Philadelphia Museum of Art on the future of foodstuffs.
Although the exhibit included bioengineered bread and genetically modified salmon, it was an installation called “Ouroboros Steak” that drew the most attention. That was comprised of pieces of human flesh grown in a lab from cultivated cells and expired blood products obtained from online sources.
The exhibit was presented as four tiny morsels of red meat – shaped in patterns suggesting an ouroboros, a dragon eating its own tail. They were placed in tiny individual saucers atop a larger plate and placemat with a calico pattern, suggesting an item to order in a diner. The artwork drew international headlines – as well as condemnation for Telhan’s vision.
Telhan’s artwork is intended to critique the overarching assumption that lab-grown meat will eventually replace more traditional production methods, as well as the lack of transparency surrounding many processed foodstuffs. “They think that this problem (from industrial-scale agriculture) is going be solved by this new technology,” Telhan said. “I am critical (of) that perspective.”
Unlike Bramble, Telhan is not against lab-grown meat, so long as its producers are transparent about the sourcing of materials and its cultivation. But he believes that large-scale agricultural meat production – which dates back centuries – is not going to be replaced so quickly.
“We see this again and again with different industries, like algae-based fuels. A lot of companies were excited about this, and promoted it,” Telhan said. “And years later, we know these fuels work. But to be able to displace the oil industry means building the infrastructure to scale takes billions of dollars, and nobody has the patience or money to do it.”
Alvaro concurred on this point, which he believes is already weakened because a large swath of consumers aren’t concerned about environmental degradation.
“They’re going to have to sell this big, but in order to convince people to do so, they have to convince them to eat this product instead of regular meat,” Alvaro said.
Hidden Tweaks?
Moreover, if lab-based meat does obtain a significant market share, Telhan suggested companies may do things to the product – such as to genetically modify it to become more profitable – and never notify consumers. That is a particular concern in the U.S., where regulations regarding such modifications are vastly more relaxed than in the European Union.
“I think that they have really good objectives, and they aspire to good objectives,” Telhan said. “But the system itself doesn't really allow for that much transparency.”
No matter what the future holds, sometime next year Carnegie Mellon is expected to hold a press conference announcing it has produced a cut of the world’s most expensive beef with the help of a modified piece of consumer electronics. It will likely take place at around the same time UPSIDE chicken will be available for purchase in supermarkets and restaurants, pending the USDA’s approvals.
Abbott, the Carnegie Mellon professor, suggested the future event will be both informative and celebratory.
“I think Carnegie Mellon would have someone potentially cook it for us,” she said. “Like have a really good chef in New York City do it.”

