Are the gains from gain-of-function research worth the risks?
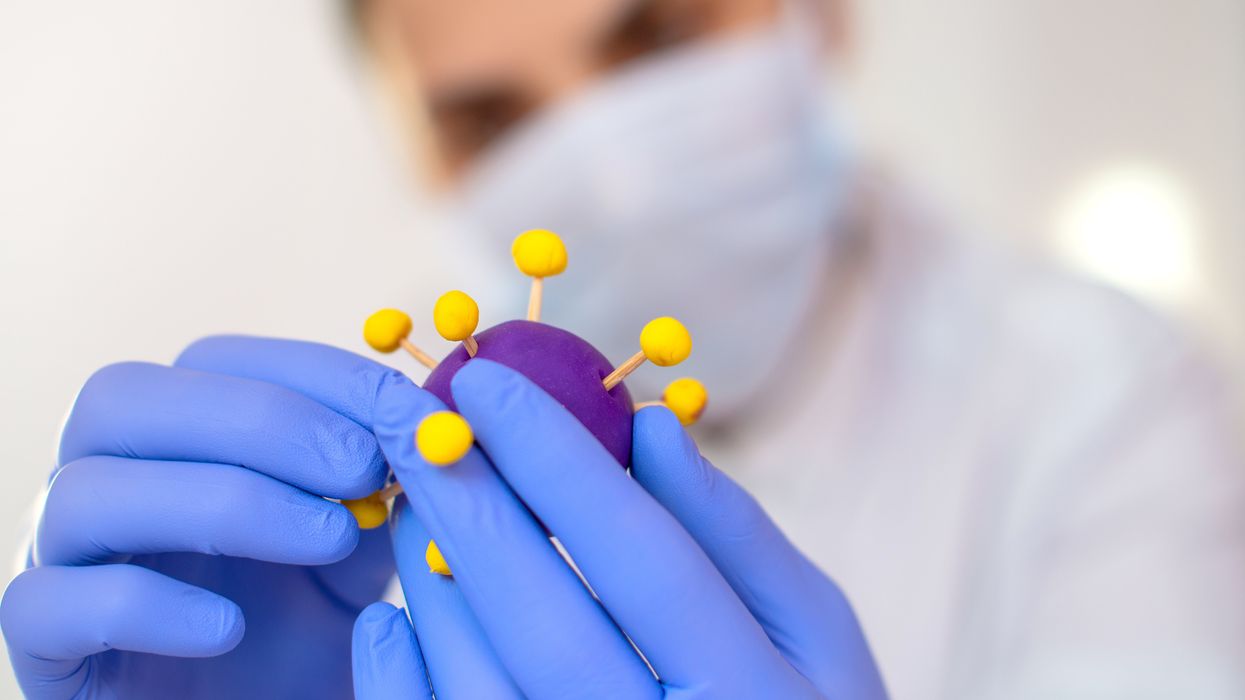
Gain-of-function research can make pathogens more infectious and deadly. It also enables scientists to prepare remedies in advance.
Scientists have long argued that gain-of-function research, which can make viruses and other infectious agents more contagious or more deadly, was necessary to develop therapies and vaccines to counter the pathogens in case they were used for biological warfare. As the SARS-CoV-2 origins are being investigated, one prominent theory suggests it had leaked from a biolab that conducted gain-of-function research, causing a global pandemic that claimed nearly 6.9 million lives. Now some question the wisdom of engaging in this type of research, stating that the risks may far outweigh the benefits.
“Gain-of-function research means genetically changing a genome in a way that might enhance the biological function of its genes, such as its transmissibility or the range of hosts it can infect,” says George Church, professor of genetics at Harvard Medical School. This can occur through direct genetic manipulation as well as by encouraging mutations while growing successive generations of micro-organism in culture. “Some of these changes may impact pathogenesis in a way that is hard to anticipate in advance,” Church says.
In the wake of the global pandemic, the pros and cons of gain-of-function research are being fiercely debated. Some scientists say this type of research is vital for preventing future pandemics or for preparing for bioweapon attacks. Others consider it another disaster waiting to happen. The Government Accounting Office issued a report charging that a framework developed by the U.S. Department of Health & Human Services (HHS) provided inadequate oversight of this potentially deadly research. There’s a movement to stop it altogether. In January, the Viral Gain-of-Function Research Moratorium Act (S. 81) was introduced into the Senate to cease awarding federal research funding to institutions doing gain-of-function studies.
While testifying before the House COVID Origins Select Committee on March 8th, Robert Redfield, former director of the U.S. Centers for Disease Control and Prevention, said that COVID-19 may have resulted from an accidental lab leak involving gain-of-function research. Redfield said his conclusion is based upon the “rapid and high infectivity for human-to-human transmission, which then predicts the rapid evolution of new variants.”
“It is a very, very, very small subset of life science research that could potentially generate a potential pandemic pathogen,” said Gerald Parker, associate dean for Global One Health at Texas A&M University.
“In my opinion,” Redfield continues, “the COVID-19 pandemic presents a case study on the potential dangers of such research. While many believe that gain-of-function research is critical to get ahead of viruses by developing vaccines, in this case, I believe that was the exact opposite.” Consequently, Redfield called for a moratorium on gain-of-function research until there is consensus about the value of such risky science.
What constitutes risky?
The Federal Select Agent Program lists 68 specific infectious agents as risky because they are either very contagious or very deadly. In order to work with these 68 agents, scientists must register with the federal government. Meanwhile, research on deadly pathogens that aren’t easily transmitted, or pathogens that are quite contagious but not deadly, can be conducted without such oversight. “If you’re not working with select agents, you’re not required to register the research with the federal government,” says Gerald Parker, associate dean for Global One Health at Texas A&M University. But the 68-item list may not have everything that could possibly become dangerous or be engineered to be dangerous, thus escaping the government’s scrutiny—an issue that new regulations aim to address.
In January 2017, the White House Office of Science and Technology Policy (OSTP) issued additional guidance. It required federal departments and agencies to follow a series of steps when reviewing proposed research that could create, transfer, or use potential pandemic pathogens resulting from the enhancement of a pathogen’s transmissibility or virulence in humans.
In defining risky pathogens, OSTP included viruses that were likely to be highly transmissible and highly virulent, and thus very deadly. The Proposed Biosecurity Oversight Framework for the Future of Science, outlined in 2023, broadened the scope to require federal review of research “that is reasonably anticipated to enhance the transmissibility and/or virulence of any pathogen” likely to pose a threat to public health, health systems or national security. Those types of experiments also include the pathogens’ ability to evade vaccines or therapeutics, or diagnostic detection.
However, Parker says that dangers of generating a pandemic-level germ are tiny. “It is a very, very, very small subset of life science research that could potentially generate a potential pandemic pathogen.” Since gain-of-function guidelines were first issued in 2017, only three such research projects have met those requirements for HHS review. They aimed to study influenza and bird flu. Only two of those projects were funded, according to the NIH Office of Science Policy. For context, NIH funded approximately 11,000 of the 54,000 grant applications it received in 2022.
Guidelines governing gain-of-function research are being strengthened, but Church points out they aren’t ideal yet. “They need to be much clearer about penalties and avoiding positive uses before they would be enforceable.”
What do we gain from gain-of-function research?
The most commonly cited reason to conduct gain-of-function research is for biodefense—the government’s ability to deal with organisms that may pose threats to public health.
In the era of mRNA vaccines, the advance preparedness argument may be even less relevant.
“The need to work with potentially dangerous viruses is central to our preparedness,” Parker says. “It’s essential that we know and understand the basic biology, microbiology, etc. of some of these dangerous pathogens.” That includes increasing our knowledge of the molecular mechanisms by which a virus could become a sustained threat to humans. “Knowing that could help us detect [risks] earlier,” Parker says—and could make it possible to have medical countermeasures, like vaccines and therapeutics, ready.
Most vaccines, however, aren’t affected by this type of research. Essentially, scientists hope they will never need to use it. Moreover, Paul Mango, HSS former deputy chief of staff for policy, and author of the 2022 book Warp Speed, says he believes that in the era of mRNA vaccines, the advance preparedness argument may be even less relevant. “That’s because these vaccines can be developed and produced in less than 12 months, unlike traditional vaccines that require years of development,” he says.
Can better oversight guarantee safety?
Another situation, which Parker calls unnecessarily dangerous, is when regulatory bodies cannot verify that the appropriate biosafety and biosecurity controls are in place.
Gain-of-function studies, Parker points out, are conducted at the basic research level, and they’re performed in high-containment labs. “As long as all the processes, procedures and protocols are followed and there’s appropriate oversight at the institutional and scientific level, it can be conducted safely.”
Globally, there are 69 Biosafety Level 4 (BSL4) labs operating, under construction or being planned, according to recent research from King’s College London and George Mason University for Global BioLabs. Eleven of these 18 high-containment facilities that are planned or under construction are in Asia. Overall, three-quarters of the BSL4 labs are in cities, increasing public health risks if leaks occur.
Researchers say they are confident in the oversight system for BSL4 labs within the U.S. They are less confident in international labs. Global BioLabs’ report concurs. It gives the highest scores for biosafety to industrialized nations, led by France, Australia, Canada, the U.S. and Japan, and the lowest scores to Saudi Arabia, India and some developing African nations. Scores for biosecurity followed similar patterns.
“There are no harmonized international biosafety and biosecurity standards,” Parker notes. That issue has been discussed for at least a decade. Now, in the wake of SARS and the COVID-19 pandemic, scientists and regulators are likely to push for unified oversight standards. “It’s time we got serious about international harmonization of biosafety and biosecurity standards and guidelines,” Parker says. New guidelines are being worked on. The National Science Advisory Board for Biosecurity (NSABB) outlined its proposed recommendations in the document titled Proposed Biosecurity Oversight Framework for the Future of Science.
The debates about whether gain-of-function research is useful or poses unnecessary risks to humanity are likely to rage on for a while. The public too has a voice in this debate and should weigh in by communicating with their representatives in government, or by partaking in educational forums or initiatives offered by universities and other institutions. In the meantime, scientists should focus on improving the research regulations, Parker notes. “We need to continue to look for lessons learned and for gaps in our oversight system,” he says. “That’s what we need to do right now.”
The future of non-hormonal birth control: Antibodies can stop sperm in their tracks
Many women want non-hormonal birth control. A 22-year-old's findings were used to launch a company that could, within the decade, bring a new kind of contraceptive to the marketplace.
Unwanted pregnancy can now be added to the list of preventions that antibodies may be fighting in the near future. For decades, really since the 1980s, engineered monoclonal antibodies have been knocking out invading germs — preventing everything from cancer to COVID. Sperm, which have some of the same properties as germs, may be next.
Not only is there an unmet need on the market for alternatives to hormonal contraceptives, the genesis for the original research was personal for the then 22-year-old scientist who led it. Her findings were used to launch a company that could, within the decade, bring a new kind of contraceptive to the marketplace.
The genesis
It’s Suruchi Shrestha’s research — published in Science Translational Medicine in August 2021 and conducted as part of her dissertation while she was a graduate student at the University of North Carolina at Chapel Hill — that could change the future of contraception for many women worldwide. According to a Guttmacher Institute report, in the U.S. alone, there were 46 million sexually active women of reproductive age (15–49) who did not want to get pregnant in 2018. With the overturning of Roe v. Wade last year, Shrestha’s research could, indeed, be life changing for millions of American women and their families.
Now a scientist with NextVivo, Shrestha is not directly involved in the development of the contraceptive that is based on her research. But, back in 2016 when she was going through her own problems with hormonal contraceptives, she “was very personally invested” in her research project, Shrestha says. She was coping with a long list of negative effects from an implanted hormonal IUD. According to the Mayo Clinic, those can include severe pelvic pain, headaches, acute acne, breast tenderness, irregular bleeding and mood swings. After a year, she had the IUD removed, but it took another full year before all the side effects finally subsided; she also watched her sister suffer the “same tribulations” after trying a hormonal IUD, she says.
For contraceptive use either daily or monthly, Shrestha says, “You want the antibody to be very potent and also cheap.” That was her goal when she launched her study.
Shrestha unshelved antibody research that had been sitting idle for decades. It was in the late 80s that scientists in Japan first tried to develop anti-sperm antibodies for contraceptive use. But, 35 years ago, “Antibody production had not been streamlined as it is now, so antibodies were very expensive,” Shrestha explains. So, they shifted away from birth control, opting to focus on developing antibodies for vaccines.
Over the course of the last three decades, different teams of researchers have been working to make the antibody more effective, bringing the cost down, though it’s still expensive, according to Shrestha. For contraceptive use either daily or monthly, she says, “You want the antibody to be very potent and also cheap.” That was her goal when she launched her study.
The problem
The problem with contraceptives for women, Shrestha says, is that all but a few of them are hormone-based or have other negative side effects. In fact, some studies and reports show that millions of women risk unintended pregnancy because of medical contraindications with hormone-based contraceptives or to avoid the risks and side effects. While there are about a dozen contraceptive choices for women, there are two for men: the condom, considered 98% effective if used correctly, and vasectomy, 99% effective. Neither of these choices are hormone-based.
On the non-hormonal side for women, there is the diaphragm which is considered only 87 percent effective. It works better with the addition of spermicides — Nonoxynol-9, or N-9 — however, they are detergents; they not only kill the sperm, they also erode the vaginal epithelium. And, there’s the non-hormonal IUD which is 99% effective. However, the IUD needs to be inserted by a medical professional, and it has a number of negative side effects, including painful cramping at a higher frequency and extremely heavy or “abnormal” and unpredictable menstrual flows.
The hormonal version of the IUD, also considered 99% effective, is the one Shrestha used which caused her two years of pain. Of course, there’s the pill, which needs to be taken daily, and the birth control ring which is worn 24/7. Both cause side effects similar to the other hormonal contraceptives on the market. The ring is considered 93% effective mostly because of user error; the pill is considered 99% effective if taken correctly.
“That’s where we saw this opening or gap for women. We want a safe, non-hormonal contraceptive,” Shrestha says. Compounding the lack of good choices, is poor access to quality sex education and family planning information, according to the non-profit Urban Institute. A focus group survey suggested that the sex education women received “often lacked substance, leaving them feeling unprepared to make smart decisions about their sexual health and safety,” wrote the authors of the Urban Institute report. In fact, nearly half (45%, or 2.8 million) of the pregnancies that occur each year in the US are unintended, reports the Guttmacher Institute. Globally the numbers are similar. According to a new report by the United Nations, each year there are 121 million unintended pregnancies, worldwide.
The science
The early work on antibodies as a contraceptive had been inspired by women with infertility. It turns out that 9 to 12 percent of women who are treated for infertility have antibodies that develop naturally and work against sperm. Shrestha was encouraged that the antibodies were specific to the target — sperm — and therefore “very safe to use in women.” She aimed to make the antibodies more stable, more effective and less expensive so they could be more easily manufactured.
Since antibodies tend to stick to things that you tell them to stick to, the idea was, basically, to engineer antibodies to stick to sperm so they would stop swimming. Shrestha and her colleagues took the binding arm of an antibody that they’d isolated from an infertile woman. Then, targeting a unique surface antigen present on human sperm, they engineered a panel of antibodies with as many as six to 10 binding arms — “almost like tongs with prongs on the tongs, that bind the sperm,” explains Shrestha. “We decided to add those grabbers on top of it, behind it. So it went from having two prongs to almost 10. And the whole goal was to have so many arms binding the sperm that it clumps it” into a “dollop,” explains Shrestha, who earned a patent on her research.

Suruchi Shrestha works in the lab with a colleague. In 2016, her research on antibodies for birth control was inspired by her own experience with side effects from an implanted hormonal IUD.
UNC - Chapel Hill
The sperm stays right where it met the antibody, never reaching the egg for fertilization. Eventually, and naturally, “Our vaginal system will just flush it out,” Shrestha explains.
“She showed in her early studies that [she] definitely got the sperm immotile, so they didn't move. And that was a really promising start,” says Jasmine Edelstein, a scientist with an expertise in antibody engineering who was not involved in this research. Shrestha’s team at UNC reproduced the effect in the sheep, notes Edelstein, who works at the startup Be Biopharma. In fact, Shrestha’s anti-sperm antibodies that caused the sperm to agglutinate, or clump together, were 99.9% effective when delivered topically to the sheep’s reproductive tracts.
The future
Going forward, Shrestha thinks the ideal approach would be delivering the antibodies through a vaginal ring. “We want to use it at the source of the spark,” Shrestha says, as opposed to less direct methods, such as taking a pill. The ring would dissolve after one month, she explains, “and then you get another one.”
Engineered to have a long shelf life, the anti-sperm antibody ring could be purchased without a prescription, and women could insert it themselves, without a doctor. “That's our hope, so that it is accessible,” Shrestha says. “Anybody can just go and grab it and not worry about pregnancy or unintended pregnancy.”
Her patented research has been licensed by several biotech companies for clinical trials. A number of Shrestha’s co-authors, including her lab advisor, Sam Lai, have launched a company, Mucommune, to continue developing the contraceptives based on these antibodies.
And, results from a small clinical trial run by researchers at Boston University Chobanian & Avedisian School of Medicine show that a dissolvable vaginal film with antibodies was safe when tested on healthy women of reproductive age. That same group of researchers last year received a $7.2 million grant from the National Institute of Health for further research on monoclonal antibody-based contraceptives, which have also been shown to block transmission of viruses, like HIV.
“As the costs come down, this becomes a more realistic option potentially for women,” says Edelstein. “The impact could be tremendous.”
This article was first published by Leaps.org in December, 2022. It has been lightly edited with updates for timeliness.
Researchers probe extreme gene therapy for severe alcoholism
When all traditional therapeutic approaches fail for alcohol abuse disorder, a radical gene therapy might be something to try in the future.
Story by Freethink
A single shot — a gene therapy injected into the brain — dramatically reduced alcohol consumption in monkeys that previously drank heavily. If the therapy is safe and effective in people, it might one day be a permanent treatment for alcoholism for people with no other options.
The challenge: Alcohol use disorder (AUD) means a person has trouble controlling their alcohol consumption, even when it is negatively affecting their life, job, or health.
In the U.S., more than 10 percent of people over the age of 12 are estimated to have AUD, and while medications, counseling, or sheer willpower can help some stop drinking, staying sober can be a huge struggle — an estimated 40-60 percent of people relapse at least once.
A team of U.S. researchers suspected that an in-development gene therapy for Parkinson’s disease might work as a dopamine-replenishing treatment for alcoholism, too.
According to the CDC, more than 140,000 Americans are dying each year from alcohol-related causes, and the rate of deaths has been rising for years, especially during the pandemic.
The idea: For occasional drinkers, alcohol causes the brain to release more dopamine, a chemical that makes you feel good. Chronic alcohol use, however, causes the brain to produce, and process, less dopamine, and this persistent dopamine deficit has been linked to alcohol relapse.
There is currently no way to reverse the changes in the brain brought about by AUD, but a team of U.S. researchers suspected that an in-development gene therapy for Parkinson’s disease might work as a dopamine-replenishing treatment for alcoholism, too.
To find out, they tested it in heavy-drinking monkeys — and the animals’ alcohol consumption dropped by 90% over the course of a year.
How it works: The treatment centers on the protein GDNF (“glial cell line-derived neurotrophic factor”), which supports the survival of certain neurons, including ones linked to dopamine.
For the new study, a harmless virus was used to deliver the gene that codes for GDNF into the brains of four monkeys that, when they had the option, drank heavily — the amount of ethanol-infused water they consumed would be equivalent to a person having nine drinks per day.
“We targeted the cell bodies that produce dopamine with this gene to increase dopamine synthesis, thereby replenishing or restoring what chronic drinking has taken away,” said co-lead researcher Kathleen Grant.
To serve as controls, another four heavy-drinking monkeys underwent the same procedure, but with a saline solution delivered instead of the gene therapy.
The results: All of the monkeys had their access to alcohol removed for two months following the surgery. When it was then reintroduced for four weeks, the heavy drinkers consumed 50 percent less compared to the control group.
When the researchers examined the monkeys’ brains at the end of the study, they were able to confirm that dopamine levels had been replenished in the treated animals, but remained low in the controls.
The researchers then took the alcohol away for another four weeks, before giving it back for four. They repeated this cycle for a year, and by the end of it, the treated monkeys’ consumption had fallen by more than 90 percent compared to the controls.
“Drinking went down to almost zero,” said Grant. “For months on end, these animals would choose to drink water and just avoid drinking alcohol altogether. They decreased their drinking to the point that it was so low we didn’t record a blood-alcohol level.”
When the researchers examined the monkeys’ brains at the end of the study, they were able to confirm that dopamine levels had been replenished in the treated animals, but remained low in the controls.
Looking ahead: Dopamine is involved in a lot more than addiction, so more research is needed to not only see if the results translate to people but whether the gene therapy leads to any unwanted changes to mood or behavior.
Because the therapy requires invasive brain surgery and is likely irreversible, it’s unlikely to ever become a common treatment for alcoholism — but it could one day be the only thing standing between people with severe AUD and death.
“[The treatment] would be most appropriate for people who have already shown that all our normal therapeutic approaches do not work for them,” said Grant. “They are likely to create severe harm or kill themselves or others due to their drinking.”
This article originally appeared on Freethink, home of the brightest minds and biggest ideas of all time.


