Scientists discover the Achilles' heel (or head) of PFAS, cancer-causing chemicals
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.

Brittany Trang led research on a new way to destroy "forever chemicals," which cause a litany of health problems, while working in William Dichtel’s chemistry lab at Northwestern University.
Brittany Trang was staring at her glass test tube, which suddenly turned opaque white. At first, she had thought that the chemical reaction she tested left behind some residue, but when she couldn’t clean it off, she realized that the reaction produced corrosive compounds that ate at the glass. That, however, was a good sign. It meant that the reaction, which she didn’t necessarily expect to work, was in fact, working. And Trang, who in 2020 was a Ph.D. researcher in chemistry at Northwestern University, had reasons to be skeptical. She was trying to break down the nearly indestructible molecules of per- and polyfluoroalkyl substances or PFAS—the forever chemicals called so because they resist heat, oil, stains, grease, and water, and thus don’t react or break down in the environment.
“The first time I ran this, I was like, oh, like there's a bunch of stuff stuck to the glass, but when I tried to clean it, it wasn’t coming off,” Trang says, recalling her original experiment and her almost-disbelief at the fact she managed to crack the notoriously stubborn and problematic molecules. “I was mostly just surprised that it worked in general.”
In the recent past, the world has been growing increasingly concerned about PFAS, the pollutants that even at low levels are associated with a litany of adverse health effects, including liver damage, thyroid disease, high cholesterol, pregnancy complications and several cancers. Used for decades in manufacturing and in various products such as fire retardant foam, water-repellant clothes, furniture fabrics, Teflon-coated pans, disposable plates, lunch containers and shoes, these super-stable compounds don’t degrade in the environment. The forever chemicals are now everywhere: in the water, in soil, in milk, and in produce.
As of June 2022, the Environmental Working Group, a nonprofit watchdog organization, found 2,858 locations in 50 states and two territories to be heavily contaminated with PFAS while many farmers had been forced to dump their milk or spinach because the levels of these compounds were in some cases up to 400 times greater than what’s considered safe. And because PFAS are so pervasive in the environment and the food we eat, they are in our bodies too. One study found some levels of PFAS in 97 to 100 percent of participants tested.
Because these compounds were made to be very stable, they are hard to destroy. So far, the only known way to break down PFAS has been to “cook” them under very harsh conditions. The process, known as pyrolysis, requires upwards of 500 degrees Centigrade, high pressure and absence of oxygen, which is energy expensive. It involves sophisticated equipment and the burning of fossil fuels. Trang, who worked in the laboratory of William Dichtel, managed to break PFAS at 120 degrees Centigrade (248 F) without using strong pressure. After she examined the results of her process with various techniques that help quantify the resulting compounds and confirmed that PFAS had indeed degraded into carbon and the corrosive fluorine that clouded her glass, she was thrilled that it worked in such simple conditions.
“That's really what differentiates our finding from everything else that's out there,” Dichtel said about their discovery at a press conference announcing the study last month. “When we're talking about low temperatures, we're at 120 degrees Celsius and sometimes even quite a bit lower than that, and especially ambient pressure.”
The process used by Trang’s team was the exact opposite of the typical organic synthesis method.
Trang’s journey into PFAS degradation began with a paper she read about the nuances of the chemicals’ molecular structure. A long molecule comprised primarily of carbon and fluorine atoms, along with oxygen and hydrogen, it has what Trang describes as a head and a tail. At the head sits a compound called carboxylic acid while the fluorine atoms make up the tail portion, with the atomic bonds so strong they aren’t possible to break without harsh treatment. But in early 2020, Trang read that a solvent called dimethylsulfoxide, or DMSO, commonly used in labs and industry, can make the carboxylic acid “pop off” its place. The DMSO doesn’t react with carboxylic acid but sort of displaces it, leaving the rest of the typically indestructible PFAS molecule vulnerable.
Trang found that its exposed fluorine tail would react with another common chemical compound, sodium hydroxide, causing a cascade of reactions that ultimately unravel the rest. “After you have decarboxylated the head, the hydroxide is able to react with the tail,” Trang says. “That's what sets off a cascade of reactions that degrades the rest of the molecule.”
That pathway took time to figure out. Trang was able to determine that the molecule carboxylic acid head popped off, but before she was able to figure out the rest, her lab and the entire Northwestern University went into lockdown in early March of 2020. “I was able to do three experiments before the shutdown,” she recalls. For the next few months, she sat at home, reading scientific literature to understand how to continue the degradation process. “I had read a bunch of literature and had a bunch of ideas for what may or may not work,” she says. By the time she could return to work, she had a plan. “I added sodium hydroxide in my batch of experiments when the lab reopened.”
The process used by Trang’s team was the exact opposite of the typical organic synthesis method. “Most organic chemists take two molecules and squish them together to make one big molecule. It’s like taking two Legos and putting them together to make one thing that was larger,” she says. “What we are doing is kind of smashing the Lego with two bits and looking at what was left to figure out how it fell apart.” The team published their discovery in the journal Science.
Although very promising, the process isn’t quite ready for industrial applications, and will take time to adapt, Trang says. For starters, it would have to be scaled up to continuously clean large quantities of water, sewage or other substances that can be contaminated with PFAS. The process will also have to be modified, particularly when it comes to removing PFAS from drinking water because as an industrial chemical, DMSO is not suitable for that. Water companies typically use activated carbon to filter out PFAS and other pollutants, so once that concentrated waste is accumulated, it would be removed and then treated with DMSO and hydroxide to break down the molecules. “That is what our method would likely be applied to,” Trang says—the concentrated waste rather than a reservoir because “you wouldn't want to mix DMSO with your drinking water.”
There are some additional limitations to the method. It only breaks down one class of forever chemicals, but there are others. For example, the molecules of perfluoroalkane sulfonic acids, or PFSA, don’t have a carboxylic head that DMSO can displace. Instead, PFSA have a sulphonic acid as their molecular head, which would require a different solvent that still needs to be discovered. “There is certainly the possibility of activating sulphonates in similar ways [to what] we've done [with] carboxylates,” Dichtel said, and he hopes this will happen in the future. Other forever chemical types may have their own Achilles’ heels, waiting to be discovered. “If we can knock that sulphonated headgroup off the molecule and get to the same intermediates we get to in this study,” Dichtel added, “it's very reasonable to assume that they'll degrade by very similar pathways.” Perhaps another team of inquisitive chemists will take on the challenge.
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.
Thanks to safety cautions from the COVID-19 pandemic, a strain of influenza has been completely eliminated.
If you were one of the millions who masked up, washed your hands thoroughly and socially distanced, pat yourself on the back—you may have helped change the course of human history.
Scientists say that thanks to these safety precautions, which were introduced in early 2020 as a way to stop transmission of the novel COVID-19 virus, a strain of influenza has been completely eliminated. This marks the first time in human history that a virus has been wiped out through non-pharmaceutical interventions, such as vaccines.
The flu shot, explained
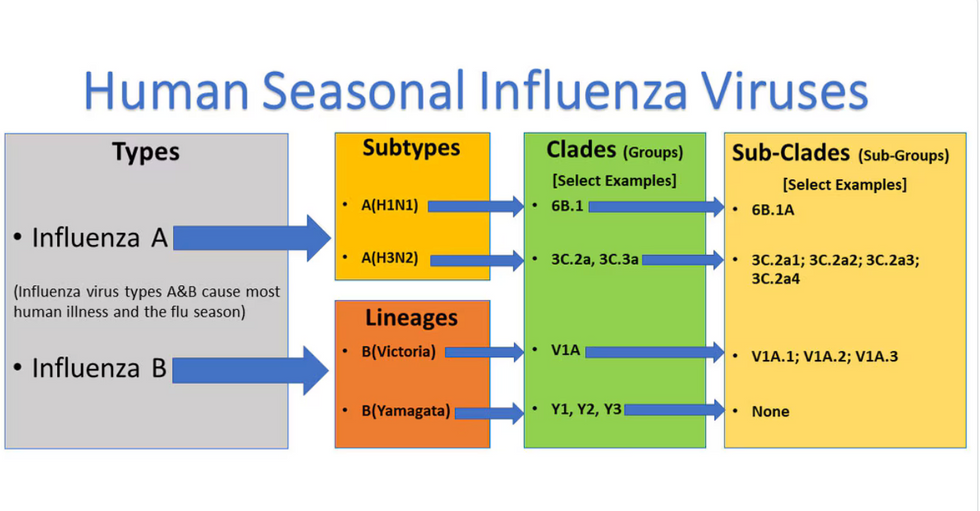
Influenza viruses type A and B are responsible for the majority of human illnesses and the flu season.
Centers for Disease Control
For more than a decade, flu shots have protected against two types of the influenza virus–type A and type B. While there are four different strains of influenza in existence (A, B, C, and D), only strains A, B, and C are capable of infecting humans, and only A and B cause pandemics. In other words, if you catch the flu during flu season, you’re most likely sick with flu type A or B.
Flu vaccines contain inactivated—or dead—influenza virus. These inactivated viruses can’t cause sickness in humans, but when administered as part of a vaccine, they teach a person’s immune system to recognize and kill those viruses when they’re encountered in the wild.
Each spring, a panel of experts gives a recommendation to the US Food and Drug Administration on which strains of each flu type to include in that year’s flu vaccine, depending on what surveillance data says is circulating and what they believe is likely to cause the most illness during the upcoming flu season. For the past decade, Americans have had access to vaccines that provide protection against two strains of influenza A and two lineages of influenza B, known as the Victoria lineage and the Yamagata lineage. But this year, the seasonal flu shot won’t include the Yamagata strain, because the Yamagata strain is no longer circulating among humans.
How Yamagata Disappeared

Flu surveillance data from the Global Initiative on Sharing All Influenza Data (GISAID) shows that the Yamagata lineage of flu type B has not been sequenced since April 2020.
Nature
Experts believe that the Yamagata lineage had already been in decline before the pandemic hit, likely because the strain was naturally less capable of infecting large numbers of people compared to the other strains. When the COVID-19 pandemic hit, the resulting safety precautions such as social distancing, isolating, hand-washing, and masking were enough to drive the virus into extinction completely.
Because the strain hasn’t been circulating since 2020, the FDA elected to remove the Yamagata strain from the seasonal flu vaccine. This will mark the first time since 2012 that the annual flu shot will be trivalent (three-component) rather than quadrivalent (four-component).
Should I still get the flu shot?
The flu shot will protect against fewer strains this year—but that doesn’t mean we should skip it. Influenza places a substantial health burden on the United States every year, responsible for hundreds of thousands of hospitalizations and tens of thousands of deaths. The flu shot has been shown to prevent millions of illnesses each year (more than six million during the 2022-2023 season). And while it’s still possible to catch the flu after getting the flu shot, studies show that people are far less likely to be hospitalized or die when they’re vaccinated.
Another unexpected benefit of dropping the Yamagata strain from the seasonal vaccine? This will possibly make production of the flu vaccine faster, and enable manufacturers to make more vaccines, helping countries who have a flu vaccine shortage and potentially saving millions more lives.
After his grandmother’s dementia diagnosis, one man invented a snack to keep her healthy and hydrated.
Founder Lewis Hornby and his grandmother Pat, sampling Jelly Drops—an edible gummy containing water and life-saving electrolytes.
On a visit to his grandmother’s nursing home in 2016, college student Lewis Hornby made a shocking discovery: Dehydration is a common (and dangerous) problem among seniors—especially those that are diagnosed with dementia.
Hornby’s grandmother, Pat, had always had difficulty keeping up her water intake as she got older, a common issue with seniors. As we age, our body composition changes, and we naturally hold less water than younger adults or children, so it’s easier to become dehydrated quickly if those fluids aren’t replenished. What’s more, our thirst signals diminish naturally as we age as well—meaning our body is not as good as it once was in letting us know that we need to rehydrate. This often creates a perfect storm that commonly leads to dehydration. In Pat’s case, her dehydration was so severe she nearly died.
When Lewis Hornby visited his grandmother at her nursing home afterward, he learned that dehydration especially affects people with dementia, as they often don’t feel thirst cues at all, or may not recognize how to use cups correctly. But while dementia patients often don’t remember to drink water, it seemed to Hornby that they had less problem remembering to eat, particularly candy.

Hornby wanted to create a solution for elderly people who struggled keeping their fluid intake up. He spent the next eighteen months researching and designing a solution and securing funding for his project. In 2019, Hornby won a sizable grant from the Alzheimer’s Society, a UK-based care and research charity for people with dementia and their caregivers. Together, through the charity’s Accelerator Program, they created a bite-sized, sugar-free, edible jelly drop that looked and tasted like candy. The candy, called Jelly Drops, contained 95% water and electrolytes—important minerals that are often lost during dehydration. The final product launched in 2020—and was an immediate success. The drops were able to provide extra hydration to the elderly, as well as help keep dementia patients safe, since dehydration commonly leads to confusion, hospitalization, and sometimes even death.
Not only did Jelly Drops quickly become a favorite snack among dementia patients in the UK, but they were able to provide an additional boost of hydration to hospital workers during the pandemic. In NHS coronavirus hospital wards, patients infected with the virus were regularly given Jelly Drops to keep their fluid levels normal—and staff members snacked on them as well, since long shifts and personal protective equipment (PPE) they were required to wear often left them feeling parched.
In April 2022, Jelly Drops launched in the United States. The company continues to donate 1% of its profits to help fund Alzheimer’s research.

