Scientists discover the Achilles' heel (or head) of PFAS, cancer-causing chemicals
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.

Brittany Trang led research on a new way to destroy "forever chemicals," which cause a litany of health problems, while working in William Dichtel’s chemistry lab at Northwestern University.
Brittany Trang was staring at her glass test tube, which suddenly turned opaque white. At first, she had thought that the chemical reaction she tested left behind some residue, but when she couldn’t clean it off, she realized that the reaction produced corrosive compounds that ate at the glass. That, however, was a good sign. It meant that the reaction, which she didn’t necessarily expect to work, was in fact, working. And Trang, who in 2020 was a Ph.D. researcher in chemistry at Northwestern University, had reasons to be skeptical. She was trying to break down the nearly indestructible molecules of per- and polyfluoroalkyl substances or PFAS—the forever chemicals called so because they resist heat, oil, stains, grease, and water, and thus don’t react or break down in the environment.
“The first time I ran this, I was like, oh, like there's a bunch of stuff stuck to the glass, but when I tried to clean it, it wasn’t coming off,” Trang says, recalling her original experiment and her almost-disbelief at the fact she managed to crack the notoriously stubborn and problematic molecules. “I was mostly just surprised that it worked in general.”
In the recent past, the world has been growing increasingly concerned about PFAS, the pollutants that even at low levels are associated with a litany of adverse health effects, including liver damage, thyroid disease, high cholesterol, pregnancy complications and several cancers. Used for decades in manufacturing and in various products such as fire retardant foam, water-repellant clothes, furniture fabrics, Teflon-coated pans, disposable plates, lunch containers and shoes, these super-stable compounds don’t degrade in the environment. The forever chemicals are now everywhere: in the water, in soil, in milk, and in produce.
As of June 2022, the Environmental Working Group, a nonprofit watchdog organization, found 2,858 locations in 50 states and two territories to be heavily contaminated with PFAS while many farmers had been forced to dump their milk or spinach because the levels of these compounds were in some cases up to 400 times greater than what’s considered safe. And because PFAS are so pervasive in the environment and the food we eat, they are in our bodies too. One study found some levels of PFAS in 97 to 100 percent of participants tested.
Because these compounds were made to be very stable, they are hard to destroy. So far, the only known way to break down PFAS has been to “cook” them under very harsh conditions. The process, known as pyrolysis, requires upwards of 500 degrees Centigrade, high pressure and absence of oxygen, which is energy expensive. It involves sophisticated equipment and the burning of fossil fuels. Trang, who worked in the laboratory of William Dichtel, managed to break PFAS at 120 degrees Centigrade (248 F) without using strong pressure. After she examined the results of her process with various techniques that help quantify the resulting compounds and confirmed that PFAS had indeed degraded into carbon and the corrosive fluorine that clouded her glass, she was thrilled that it worked in such simple conditions.
“That's really what differentiates our finding from everything else that's out there,” Dichtel said about their discovery at a press conference announcing the study last month. “When we're talking about low temperatures, we're at 120 degrees Celsius and sometimes even quite a bit lower than that, and especially ambient pressure.”
The process used by Trang’s team was the exact opposite of the typical organic synthesis method.
Trang’s journey into PFAS degradation began with a paper she read about the nuances of the chemicals’ molecular structure. A long molecule comprised primarily of carbon and fluorine atoms, along with oxygen and hydrogen, it has what Trang describes as a head and a tail. At the head sits a compound called carboxylic acid while the fluorine atoms make up the tail portion, with the atomic bonds so strong they aren’t possible to break without harsh treatment. But in early 2020, Trang read that a solvent called dimethylsulfoxide, or DMSO, commonly used in labs and industry, can make the carboxylic acid “pop off” its place. The DMSO doesn’t react with carboxylic acid but sort of displaces it, leaving the rest of the typically indestructible PFAS molecule vulnerable.
Trang found that its exposed fluorine tail would react with another common chemical compound, sodium hydroxide, causing a cascade of reactions that ultimately unravel the rest. “After you have decarboxylated the head, the hydroxide is able to react with the tail,” Trang says. “That's what sets off a cascade of reactions that degrades the rest of the molecule.”
That pathway took time to figure out. Trang was able to determine that the molecule carboxylic acid head popped off, but before she was able to figure out the rest, her lab and the entire Northwestern University went into lockdown in early March of 2020. “I was able to do three experiments before the shutdown,” she recalls. For the next few months, she sat at home, reading scientific literature to understand how to continue the degradation process. “I had read a bunch of literature and had a bunch of ideas for what may or may not work,” she says. By the time she could return to work, she had a plan. “I added sodium hydroxide in my batch of experiments when the lab reopened.”
The process used by Trang’s team was the exact opposite of the typical organic synthesis method. “Most organic chemists take two molecules and squish them together to make one big molecule. It’s like taking two Legos and putting them together to make one thing that was larger,” she says. “What we are doing is kind of smashing the Lego with two bits and looking at what was left to figure out how it fell apart.” The team published their discovery in the journal Science.
Although very promising, the process isn’t quite ready for industrial applications, and will take time to adapt, Trang says. For starters, it would have to be scaled up to continuously clean large quantities of water, sewage or other substances that can be contaminated with PFAS. The process will also have to be modified, particularly when it comes to removing PFAS from drinking water because as an industrial chemical, DMSO is not suitable for that. Water companies typically use activated carbon to filter out PFAS and other pollutants, so once that concentrated waste is accumulated, it would be removed and then treated with DMSO and hydroxide to break down the molecules. “That is what our method would likely be applied to,” Trang says—the concentrated waste rather than a reservoir because “you wouldn't want to mix DMSO with your drinking water.”
There are some additional limitations to the method. It only breaks down one class of forever chemicals, but there are others. For example, the molecules of perfluoroalkane sulfonic acids, or PFSA, don’t have a carboxylic head that DMSO can displace. Instead, PFSA have a sulphonic acid as their molecular head, which would require a different solvent that still needs to be discovered. “There is certainly the possibility of activating sulphonates in similar ways [to what] we've done [with] carboxylates,” Dichtel said, and he hopes this will happen in the future. Other forever chemical types may have their own Achilles’ heels, waiting to be discovered. “If we can knock that sulphonated headgroup off the molecule and get to the same intermediates we get to in this study,” Dichtel added, “it's very reasonable to assume that they'll degrade by very similar pathways.” Perhaps another team of inquisitive chemists will take on the challenge.
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.
Urinary tract infections account for more than 8 million trips to the doctor each year.
Few things are more painful than a urinary tract infection (UTI). Common in men and women, these infections account for more than 8 million trips to the doctor each year and can cause an array of uncomfortable symptoms, from a burning feeling during urination to fever, vomiting, and chills. For an unlucky few, UTIs can be chronic—meaning that, despite treatment, they just keep coming back.
But new research, presented at the European Association of Urology (EAU) Congress in Paris this week, brings some hope to people who suffer from UTIs.
Clinicians from the Royal Berkshire Hospital presented the results of a long-term, nine-year clinical trial where 89 men and women who suffered from recurrent UTIs were given an oral vaccine called MV140, designed to prevent the infections. Every day for three months, the participants were given two sprays of the vaccine (flavored to taste like pineapple) and then followed over the course of nine years. Clinicians analyzed medical records and asked the study participants about symptoms to check whether any experienced UTIs or had any adverse reactions from taking the vaccine.
The results showed that across nine years, 48 of the participants (about 54%) remained completely infection-free. On average, the study participants remained infection free for 54.7 months—four and a half years.
“While we need to be pragmatic, this vaccine is a potential breakthrough in preventing UTIs and could offer a safe and effective alternative to conventional treatments,” said Gernot Bonita, Professor of Urology at the Alta Bro Medical Centre for Urology in Switzerland, who is also the EAU Chairman of Guidelines on Urological Infections.
The news comes as a relief not only for people who suffer chronic UTIs, but also to doctors who have seen an uptick in antibiotic-resistant UTIs in the past several years. Because UTIs usually require antibiotics, patients run the risk of developing a resistance to the antibiotics, making infections more difficult to treat. A preventative vaccine could mean less infections, less antibiotics, and less drug resistance overall.
“Many of our participants told us that having the vaccine restored their quality of life,” said Dr. Bob Yang, Consultant Urologist at the Royal Berkshire NHS Foundation Trust, who helped lead the research. “While we’re yet to look at the effect of this vaccine in different patient groups, this follow-up data suggests it could be a game-changer for UTI prevention if it’s offered widely, reducing the need for antibiotic treatments.”
MILESTONE: Doctors have transplanted a pig organ into a human for the first time in history
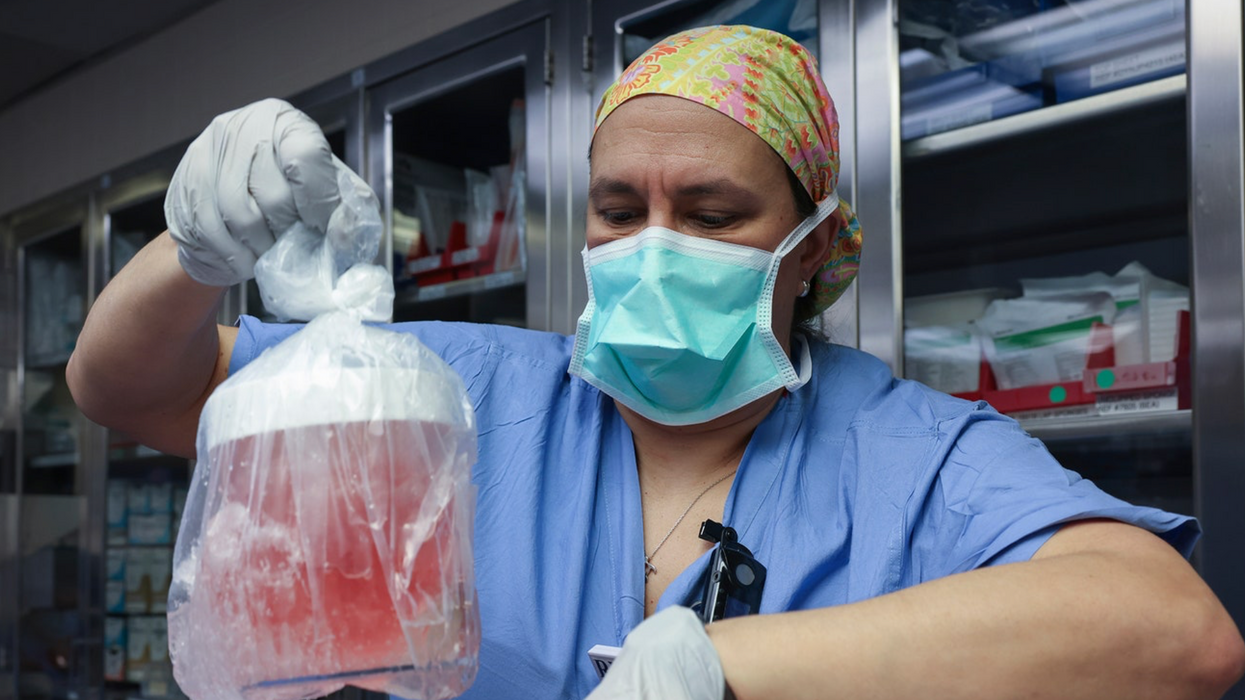
A surgeon at Massachusetts General Hospital prepares a pig organ for transplant.
Surgeons at Massachusetts General Hospital made history last week when they successfully transplanted a pig kidney into a human patient for the first time ever.
The recipient was a 62-year-old man named Richard Slayman who had been living with end-stage kidney disease caused by diabetes. While Slayman had received a kidney transplant in 2018 from a human donor, his diabetes ultimately caused the kidney to fail less than five years after the transplant. Slayman had undergone dialysis ever since—a procedure that uses an artificial kidney to remove waste products from a person’s blood when the kidneys are unable to—but the dialysis frequently caused blood clots and other complications that landed him in the hospital multiple times.
As a last resort, Slayman’s kidney specialist suggested a transplant using a pig kidney provided by eGenesis, a pharmaceutical company based in Cambridge, Mass. The highly experimental surgery was made possible with the Food and Drug Administration’s “compassionate use” initiative, which allows patients with life-threatening medical conditions access to experimental treatments.
The new frontier of organ donation
Like Slayman, more than 100,000 people are currently on the national organ transplant waiting list, and roughly 17 people die every day waiting for an available organ. To make up for the shortage of human organs, scientists have been experimenting for the past several decades with using organs from animals such as pigs—a new field of medicine known as xenotransplantation. But putting an animal organ into a human body is much more complicated than it might appear, experts say.
“The human immune system reacts incredibly violently to a pig organ, much more so than a human organ,” said Dr. Joren Madsen, director of the Mass General Transplant Center. Even with immunosuppressant drugs that suppress the body’s ability to reject the transplant organ, Madsen said, a human body would reject an animal organ “within minutes.”
So scientists have had to use gene-editing technology to change the animal organs so that they would work inside a human body. The pig kidney in Slayman’s surgery, for instance, had been genetically altered using CRISPR-Cas9 technology to remove harmful pig genes and add human ones. The kidney was also edited to remove pig viruses that could potentially infect a human after transplant.
With CRISPR technology, scientists have been able to prove that interspecies organ transplants are not only possible, but may be able to successfully work long term, too. In the past several years, scientists were able to transplant a pig kidney into a monkey and have the monkey survive for more than two years. More recently, doctors have transplanted pig hearts into human beings—though each recipient of a pig heart only managed to live a couple of months after the transplant. In one of the patients, researchers noted evidence of a pig virus in the man’s heart that had not been identified before the surgery and could be a possible explanation for his heart failure.
So far, so good
Slayman and his medical team ultimately decided to pursue the surgery—and the risk paid off. When the pig organ started producing urine at the end of the four-hour surgery, the entire operating room erupted in applause.
Slayman is currently receiving an infusion of immunosuppressant drugs to prevent the kidney from being rejected, while his doctors monitor the kidney’s function with frequent ultrasounds. Slayman is reported to be “recovering well” at Massachusetts General Hospital and is expected to be discharged within the next several days.