Scientists discover the Achilles' heel (or head) of PFAS, cancer-causing chemicals
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.

Brittany Trang led research on a new way to destroy "forever chemicals," which cause a litany of health problems, while working in William Dichtel’s chemistry lab at Northwestern University.
Brittany Trang was staring at her glass test tube, which suddenly turned opaque white. At first, she had thought that the chemical reaction she tested left behind some residue, but when she couldn’t clean it off, she realized that the reaction produced corrosive compounds that ate at the glass. That, however, was a good sign. It meant that the reaction, which she didn’t necessarily expect to work, was in fact, working. And Trang, who in 2020 was a Ph.D. researcher in chemistry at Northwestern University, had reasons to be skeptical. She was trying to break down the nearly indestructible molecules of per- and polyfluoroalkyl substances or PFAS—the forever chemicals called so because they resist heat, oil, stains, grease, and water, and thus don’t react or break down in the environment.
“The first time I ran this, I was like, oh, like there's a bunch of stuff stuck to the glass, but when I tried to clean it, it wasn’t coming off,” Trang says, recalling her original experiment and her almost-disbelief at the fact she managed to crack the notoriously stubborn and problematic molecules. “I was mostly just surprised that it worked in general.”
In the recent past, the world has been growing increasingly concerned about PFAS, the pollutants that even at low levels are associated with a litany of adverse health effects, including liver damage, thyroid disease, high cholesterol, pregnancy complications and several cancers. Used for decades in manufacturing and in various products such as fire retardant foam, water-repellant clothes, furniture fabrics, Teflon-coated pans, disposable plates, lunch containers and shoes, these super-stable compounds don’t degrade in the environment. The forever chemicals are now everywhere: in the water, in soil, in milk, and in produce.
As of June 2022, the Environmental Working Group, a nonprofit watchdog organization, found 2,858 locations in 50 states and two territories to be heavily contaminated with PFAS while many farmers had been forced to dump their milk or spinach because the levels of these compounds were in some cases up to 400 times greater than what’s considered safe. And because PFAS are so pervasive in the environment and the food we eat, they are in our bodies too. One study found some levels of PFAS in 97 to 100 percent of participants tested.
Because these compounds were made to be very stable, they are hard to destroy. So far, the only known way to break down PFAS has been to “cook” them under very harsh conditions. The process, known as pyrolysis, requires upwards of 500 degrees Centigrade, high pressure and absence of oxygen, which is energy expensive. It involves sophisticated equipment and the burning of fossil fuels. Trang, who worked in the laboratory of William Dichtel, managed to break PFAS at 120 degrees Centigrade (248 F) without using strong pressure. After she examined the results of her process with various techniques that help quantify the resulting compounds and confirmed that PFAS had indeed degraded into carbon and the corrosive fluorine that clouded her glass, she was thrilled that it worked in such simple conditions.
“That's really what differentiates our finding from everything else that's out there,” Dichtel said about their discovery at a press conference announcing the study last month. “When we're talking about low temperatures, we're at 120 degrees Celsius and sometimes even quite a bit lower than that, and especially ambient pressure.”
The process used by Trang’s team was the exact opposite of the typical organic synthesis method.
Trang’s journey into PFAS degradation began with a paper she read about the nuances of the chemicals’ molecular structure. A long molecule comprised primarily of carbon and fluorine atoms, along with oxygen and hydrogen, it has what Trang describes as a head and a tail. At the head sits a compound called carboxylic acid while the fluorine atoms make up the tail portion, with the atomic bonds so strong they aren’t possible to break without harsh treatment. But in early 2020, Trang read that a solvent called dimethylsulfoxide, or DMSO, commonly used in labs and industry, can make the carboxylic acid “pop off” its place. The DMSO doesn’t react with carboxylic acid but sort of displaces it, leaving the rest of the typically indestructible PFAS molecule vulnerable.
Trang found that its exposed fluorine tail would react with another common chemical compound, sodium hydroxide, causing a cascade of reactions that ultimately unravel the rest. “After you have decarboxylated the head, the hydroxide is able to react with the tail,” Trang says. “That's what sets off a cascade of reactions that degrades the rest of the molecule.”
That pathway took time to figure out. Trang was able to determine that the molecule carboxylic acid head popped off, but before she was able to figure out the rest, her lab and the entire Northwestern University went into lockdown in early March of 2020. “I was able to do three experiments before the shutdown,” she recalls. For the next few months, she sat at home, reading scientific literature to understand how to continue the degradation process. “I had read a bunch of literature and had a bunch of ideas for what may or may not work,” she says. By the time she could return to work, she had a plan. “I added sodium hydroxide in my batch of experiments when the lab reopened.”
The process used by Trang’s team was the exact opposite of the typical organic synthesis method. “Most organic chemists take two molecules and squish them together to make one big molecule. It’s like taking two Legos and putting them together to make one thing that was larger,” she says. “What we are doing is kind of smashing the Lego with two bits and looking at what was left to figure out how it fell apart.” The team published their discovery in the journal Science.
Although very promising, the process isn’t quite ready for industrial applications, and will take time to adapt, Trang says. For starters, it would have to be scaled up to continuously clean large quantities of water, sewage or other substances that can be contaminated with PFAS. The process will also have to be modified, particularly when it comes to removing PFAS from drinking water because as an industrial chemical, DMSO is not suitable for that. Water companies typically use activated carbon to filter out PFAS and other pollutants, so once that concentrated waste is accumulated, it would be removed and then treated with DMSO and hydroxide to break down the molecules. “That is what our method would likely be applied to,” Trang says—the concentrated waste rather than a reservoir because “you wouldn't want to mix DMSO with your drinking water.”
There are some additional limitations to the method. It only breaks down one class of forever chemicals, but there are others. For example, the molecules of perfluoroalkane sulfonic acids, or PFSA, don’t have a carboxylic head that DMSO can displace. Instead, PFSA have a sulphonic acid as their molecular head, which would require a different solvent that still needs to be discovered. “There is certainly the possibility of activating sulphonates in similar ways [to what] we've done [with] carboxylates,” Dichtel said, and he hopes this will happen in the future. Other forever chemical types may have their own Achilles’ heels, waiting to be discovered. “If we can knock that sulphonated headgroup off the molecule and get to the same intermediates we get to in this study,” Dichtel added, “it's very reasonable to assume that they'll degrade by very similar pathways.” Perhaps another team of inquisitive chemists will take on the challenge.
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.
Dr. May Edward Chinn, Kizzmekia Corbett, PhD., and Alice Ball, among others, have been behind some of the most important scientific work of the last century.
If you look back on the last century of scientific achievements, you might notice that most of the scientists we celebrate are overwhelmingly white, while scientists of color take a backseat. Since the Nobel Prize was introduced in 1901, for example, no black scientists have landed this prestigious award.
The work of black women scientists has gone unrecognized in particular. Their work uncredited and often stolen, black women have nevertheless contributed to some of the most important advancements of the last 100 years, from the polio vaccine to GPS.
Here are five black women who have changed science forever.
Dr. May Edward Chinn
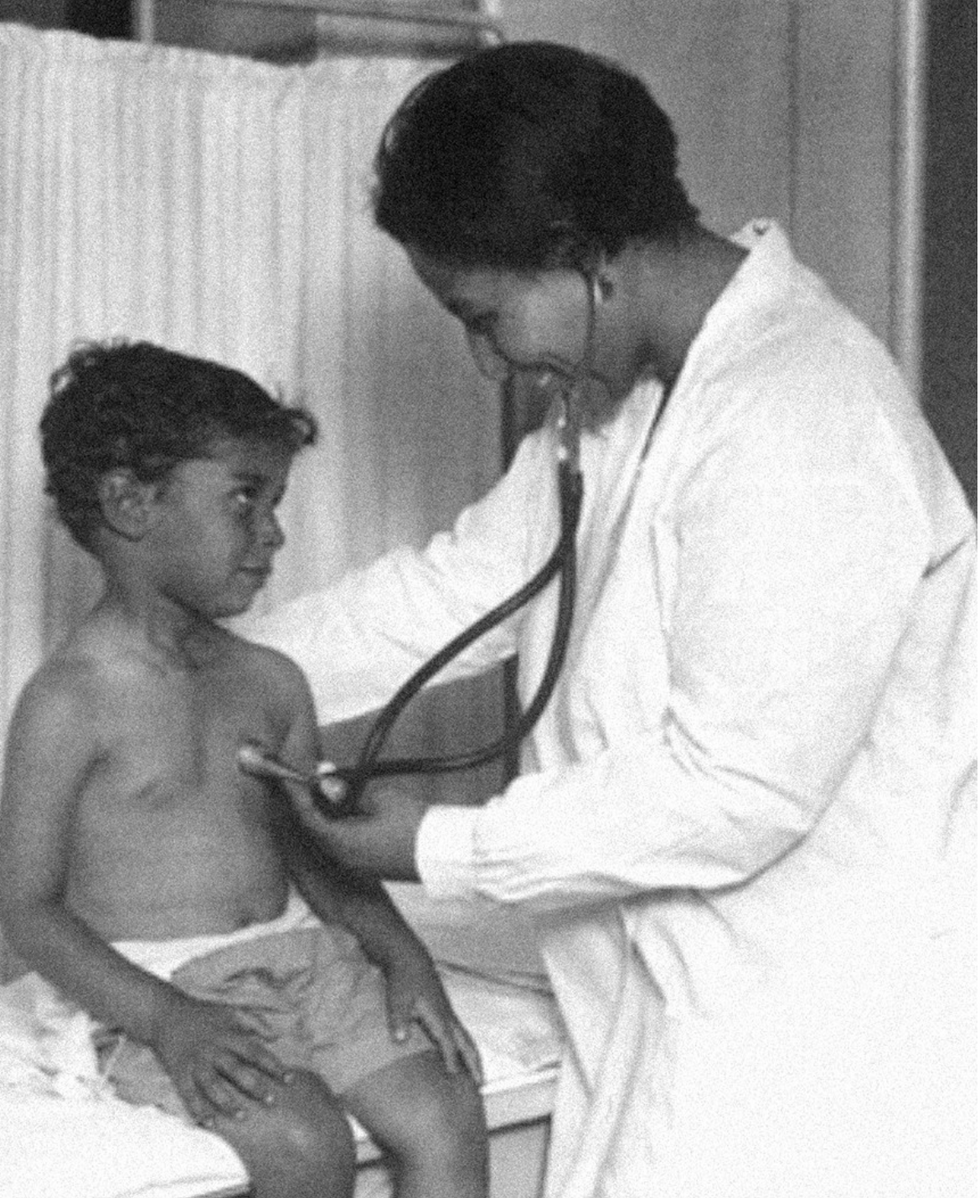
Dr. May Edward Chinn practicing medicine in Harlem
George B. Davis, PhD.
Chinn was born to poor parents in New York City just before the start of the 20th century. Although she showed great promise as a pianist, playing with the legendary musician Paul Robeson throughout the 1920s, she decided to study medicine instead. Chinn, like other black doctors of the time, were barred from studying or practicing in New York hospitals. So Chinn formed a private practice and made house calls, sometimes operating in patients’ living rooms, using an ironing board as a makeshift operating table.
Chinn worked among the city’s poor, and in doing this, started to notice her patients had late-stage cancers that often had gone undetected or untreated for years. To learn more about cancer and its prevention, Chinn begged information off white doctors who were willing to share with her, and even accompanied her patients to other clinic appointments in the city, claiming to be the family physician. Chinn took this information and integrated it into her own practice, creating guidelines for early cancer detection that were revolutionary at the time—for instance, checking patient health histories, checking family histories, performing routine pap smears, and screening patients for cancer even before they showed symptoms. For years, Chinn was the only black female doctor working in Harlem, and she continued to work closely with the poor and advocate for early cancer screenings until she retired at age 81.
Alice Ball

Pictorial Press Ltd/Alamy
Alice Ball was a chemist best known for her groundbreaking work on the development of the “Ball Method,” the first successful treatment for those suffering from leprosy during the early 20th century.
In 1916, while she was an undergraduate student at the University of Hawaii, Ball studied the effects of Chaulmoogra oil in treating leprosy. This oil was a well-established therapy in Asian countries, but it had such a foul taste and led to such unpleasant side effects that many patients refused to take it.
So Ball developed a method to isolate and extract the active compounds from Chaulmoogra oil to create an injectable medicine. This marked a significant breakthrough in leprosy treatment and became the standard of care for several decades afterward.
Unfortunately, Ball died before she could publish her results, and credit for this discovery was given to another scientist. One of her colleagues, however, was able to properly credit her in a publication in 1922.
Henrietta Lacks

onathan Newton/The Washington Post/Getty
The person who arguably contributed the most to scientific research in the last century, surprisingly, wasn’t even a scientist. Henrietta Lacks was a tobacco farmer and mother of five children who lived in Maryland during the 1940s. In 1951, Lacks visited Johns Hopkins Hospital where doctors found a cancerous tumor on her cervix. Before treating the tumor, the doctor who examined Lacks clipped two small samples of tissue from Lacks’ cervix without her knowledge or consent—something unthinkable today thanks to informed consent practices, but commonplace back then.
As Lacks underwent treatment for her cancer, her tissue samples made their way to the desk of George Otto Gey, a cancer researcher at Johns Hopkins. He noticed that unlike the other cell cultures that came into his lab, Lacks’ cells grew and multiplied instead of dying out. Lacks’ cells were “immortal,” meaning that because of a genetic defect, they were able to reproduce indefinitely as long as certain conditions were kept stable inside the lab.
Gey started shipping Lacks’ cells to other researchers across the globe, and scientists were thrilled to have an unlimited amount of sturdy human cells with which to experiment. Long after Lacks died of cervical cancer in 1951, her cells continued to multiply and scientists continued to use them to develop cancer treatments, to learn more about HIV/AIDS, to pioneer fertility treatments like in vitro fertilization, and to develop the polio vaccine. To this day, Lacks’ cells have saved an estimated 10 million lives, and her family is beginning to get the compensation and recognition that Henrietta deserved.
Dr. Gladys West

Andre West
Gladys West was a mathematician who helped invent something nearly everyone uses today. West started her career in the 1950s at the Naval Surface Warfare Center Dahlgren Division in Virginia, and took data from satellites to create a mathematical model of the Earth’s shape and gravitational field. This important work would lay the groundwork for the technology that would later become the Global Positioning System, or GPS. West’s work was not widely recognized until she was honored by the US Air Force in 2018.
Dr. Kizzmekia "Kizzy" Corbett

TIME Magazine
At just 35 years old, immunologist Kizzmekia “Kizzy” Corbett has already made history. A viral immunologist by training, Corbett studied coronaviruses at the National Institutes of Health (NIH) and researched possible vaccines for coronaviruses such as SARS (Severe Acute Respiratory Syndrome) and MERS (Middle East Respiratory Syndrome).
At the start of the COVID pandemic, Corbett and her team at the NIH partnered with pharmaceutical giant Moderna to develop an mRNA-based vaccine against the virus. Corbett’s previous work with mRNA and coronaviruses was vital in developing the vaccine, which became one of the first to be authorized for emergency use in the United States. The vaccine, along with others, is responsible for saving an estimated 14 million lives.On today’s episode of Making Sense of Science, I’m honored to be joined by Dr. Paul Song, a physician, oncologist, progressive activist and biotech chief medical officer. Through his company, NKGen Biotech, Dr. Song is leveraging the power of patients’ own immune systems by supercharging the body’s natural killer cells to make new treatments for Alzheimer’s and cancer.
Whereas other treatments for Alzheimer’s focus directly on reducing the build-up of proteins in the brain such as amyloid and tau in patients will mild cognitive impairment, NKGen is seeking to help patients that much of the rest of the medical community has written off as hopeless cases, those with late stage Alzheimer’s. And in small studies, NKGen has shown remarkable results, even improvement in the symptoms of people with these very progressed forms of Alzheimer’s, above and beyond slowing down the disease.
In the realm of cancer, Dr. Song is similarly setting his sights on another group of patients for whom treatment options are few and far between: people with solid tumors. Whereas some gradual progress has been made in treating blood cancers such as certain leukemias in past few decades, solid tumors have been even more of a challenge. But Dr. Song’s approach of using natural killer cells to treat solid tumors is promising. You may have heard of CAR-T, which uses genetic engineering to introduce cells into the body that have a particular function to help treat a disease. NKGen focuses on other means to enhance the 40 plus receptors of natural killer cells, making them more receptive and sensitive to picking out cancer cells.
Paul Y. Song, MD is currently CEO and Vice Chairman of NKGen Biotech. Dr. Song’s last clinical role was Asst. Professor at the Samuel Oschin Cancer Center at Cedars Sinai Medical Center.
Dr. Song served as the very first visiting fellow on healthcare policy in the California Department of Insurance in 2013. He is currently on the advisory board of the Pritzker School of Molecular Engineering at the University of Chicago and a board member of Mercy Corps, The Center for Health and Democracy, and Gideon’s Promise.
Dr. Song graduated with honors from the University of Chicago and received his MD from George Washington University. He completed his residency in radiation oncology at the University of Chicago where he served as Chief Resident and did a brachytherapy fellowship at the Institute Gustave Roussy in Villejuif, France. He was also awarded an ASTRO research fellowship in 1995 for his research in radiation inducible gene therapy.
With Dr. Song’s leadership, NKGen Biotech’s work on natural killer cells represents cutting-edge science leading to key findings and important pieces of the puzzle for treating two of humanity’s most intractable diseases.
Show links
- Paul Song LinkedIn
- NKGen Biotech on Twitter - @NKGenBiotech
- NKGen Website: https://nkgenbiotech.com/
- NKGen appoints Paul Song
- Patient Story: https://pix11.com/news/local-news/long-island/promising-new-treatment-for-advanced-alzheimers-patients/
- FDA Clearance: https://nkgenbiotech.com/nkgen-biotech-receives-ind-clearance-from-fda-for-snk02-allogeneic-natural-killer-cell-therapy-for-solid-tumors/Q3 earnings data: https://www.nasdaq.com/press-release/nkgen-biotech-inc.-reports-third-quarter-2023-financial-results-and-business