A surprising weapon in the fight against food poisoning
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.

Phages, which are harmless viruses that destroy specific bacteria, are becoming useful tools to protect our food supply.
Every year, one in seven people in America comes down with a foodborne illness, typically caused by a bacterial pathogen, including E.Coli, listeria, salmonella, or campylobacter. That adds up to 48 million people, of which 120,000 are hospitalized and 3000 die, according to the Centers for Disease Control. And the variety of foods that can be contaminated with bacterial pathogens is growing too. In the 20th century, E.Coli and listeria lurked primarily within meat. Now they find their way into lettuce, spinach, and other leafy greens, causing periodic consumer scares and product recalls. Onions are the most recent suspected culprit of a nationwide salmonella outbreak.
Some of these incidents are almost inevitable because of how Mother Nature works, explains Divya Jaroni, associate professor of animal and food sciences at Oklahoma State University. These common foodborne pathogens come from the cattle's intestines when the animals shed them in their manure—and then they get washed into rivers and lakes, especially in heavy rains. When this water is later used to irrigate produce farms, the bugs end up on salad greens. Plus, many small farms do both—herd cattle and grow produce.
"Unfortunately for us, these pathogens are part of the microflora of the cows' intestinal tract," Jaroni says. "Some farmers may have an acre or two of cattle pastures, and an acre of a produce farm nearby, so it's easy for this water to contaminate the crops."
Food producers and packagers fight bacteria by potent chemicals, with chlorine being the go-to disinfectant. Cattle carcasses, for example, are typically washed by chlorine solutions as the animals' intestines are removed. Leafy greens are bathed in water with added chlorine solutions. However, because the same "bath" can be used for multiple veggie batches and chlorine evaporates over time, the later rounds may not kill all of the bacteria, sparing some. The natural and organic producers avoid chlorine, substituting it with lactic acid, a more holistic sanitizer, but even with all these efforts, some pathogens survive, sickening consumers and causing food recalls. As we farm more animals and grow more produce, while also striving to use fewer chemicals and more organic growing methods, it will be harder to control bacteria's spread.
"It took us a long time to convince the FDA phages were safe and efficient alternatives. But we had worked with them to gather all the data they needed, and the FDA was very supportive in the end."
Luckily, bacteria have their own killers. Called bacteriophages, or phages for short, they are viruses that prey on bacteria only. Under the electron microscope, they look like fantasy spaceships, with oblong bodies, spider-like legs and long tails. Much smaller than a bacterium, phages pierce the microbes' cells with their tails, sneak in and begin multiplying inside, eventually bursting the microbes open—and then proceed to infect more of them.
The best part is that these phages are harmless to humans. Moreover, recent research finds that millions of phages dwell on us and in us—in our nose, throat, skin and gut, protecting us from bacterial infections as part of our healthy microbiome. A recent study suggested that we absorb about 30 billion phages into our bodies on a daily basis. Now, ingeniously, they are starting to be deployed as anti-microbial agents in the food industry.
A Maryland-based phage research company called Intralytix is doing just that. Founded by Alexander Sulakvelidze, a microbiologist and epidemiologist who came to the United States from Tbilisi, the capital of Georgia, Intralytix makes and sells five different FDA-approved phage cocktails that work against some of the most notorious food pathogens: ListShield for Listeria, SalmoFresh for Salmonella, ShigaShield for Shigella, another foodborne bug, and EcoShield for E.coli, including the infamous strain that caused the Jack in the Box outbreak in 1993 that killed four children and sickened 732 people across four states. Last year, the FDA granted its approval to yet another Intralytix phage for managing Campylobacter contamination, named CampyShield. "We call it safety by nature," Sulakvelidze says.

Intralytix grows phages inside massive 1500-liter fermenters, feeding them bacterial "fodder."
Photo credit: Living Radiant Photography
Phage preparations are relatively straightforward to make. In nature, phages thrive in any body of water where bacteria live too, including rivers, lakes and bays. "I can dip a bucket into the Chesapeake Bay, and it will be full of all kinds of phages," Sulakvelidze says. "Sewage is another great place to look for specific phages of interest, because it's teeming with all sorts of bacteria—and therefore the viruses that prey on them."
In lab settings, Intralytix grows phages inside massive 1500-liter fermenters, feeding them bacterial "fodder." Once phages multiply enough, they are harvested, dispensed into containers and shipped to food producers who have adopted this disinfecting practice into their preparation process. Typically, it's done by computer-controlled sprayer systems that disperse mist-like phage preparations onto the food.
Unlike chemicals like chlorine or antibiotics, which kill a wide spectrum of bacteria, phages are more specialized, each feeding on specific microbial species. A phage that targets salmonella will not prey on listeria and vice versa. So food producers may sometimes use a combo of different phage preparations. Intralytix is continuously researching and testing new phages. With a contract from the National Institutes of Health, Intralytix is expanding its automated high-throughput robot that tests which phages work best against which bacteria, speeding up the development of the new phage cocktails.
Phages have other "talents." In her recent study, Jaroni found that phages have the ability to destroy bacterial biofilms—colonies of microorganisms that tend to grow on surfaces of the food processing equipment, surrounding themselves with protective coating that even very harsh chemicals can't crack.
"Phages are very clever," Jaroni says. "They produce enzymes that target the biofilms, and once they break through, they can reach the bacteria."
Convincing the FDA that phages were safe to use on food products was no easy feat, Sulakvelidze says. In his home country of Georgia, phages have been used as antimicrobial remedies for over a century, but the FDA was leery of using viruses as food safety agents. "It took us a long time to convince the FDA phages were safe and efficient alternatives," Sulakvelidze says. "But we had worked with them to gather all the data they needed, and the FDA was very supportive in the end."
The agency had granted Intralytix its first approval in 2006, and over the past 10 years, the company's sales increased by over 15-fold. "We currently sell to about 40 companies and are in discussions with several other large food producers," Sulakvelidze says. One indicator that the industry now understands and appreciates the science of phages was that his company was ranked as Top Food Safety Provider in 2021 by Food and Beverage Technology Review, he adds. Notably, phage sprays are kosher, halal and organic-certified.
Intralytix's phage cocktails to safeguard food from bacteria are approved for consumers in addition to food producers, but currently the company sells to food producers only. Selling retail requires different packaging like easy-to-use spray bottles and different marketing that would inform people about phages' antimicrobial qualities. But ultimately, giving people the ability to remove pathogens from their food with probiotic phage sprays is the goal, Sulakvelidze says.
It's not the company's only goal. Now Intralytix is going a step further, investigating phages' probiotic and therapeutic abilities. Because phages are highly specialized in the bacteria they target, they can be used to treat infections caused by specific pathogens while leaving the beneficial species of our microbiome intact. In an ongoing clinical trial with Mount Sinai, Intralytix is now investigating a potential phage treatment against a certain type of E. coli for patients with Crohn's disease, and is about to start another clinical trial for treating bacterial dysentery.
"Now that we have proved that phages are safe and effective against foodborne bacteria," Sulakvelidze says, "we are going to demonstrate their potential in therapeutic applications."
This article was first published by Leaps.org on October 27, 2021.
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.
Why you should (virtually) care
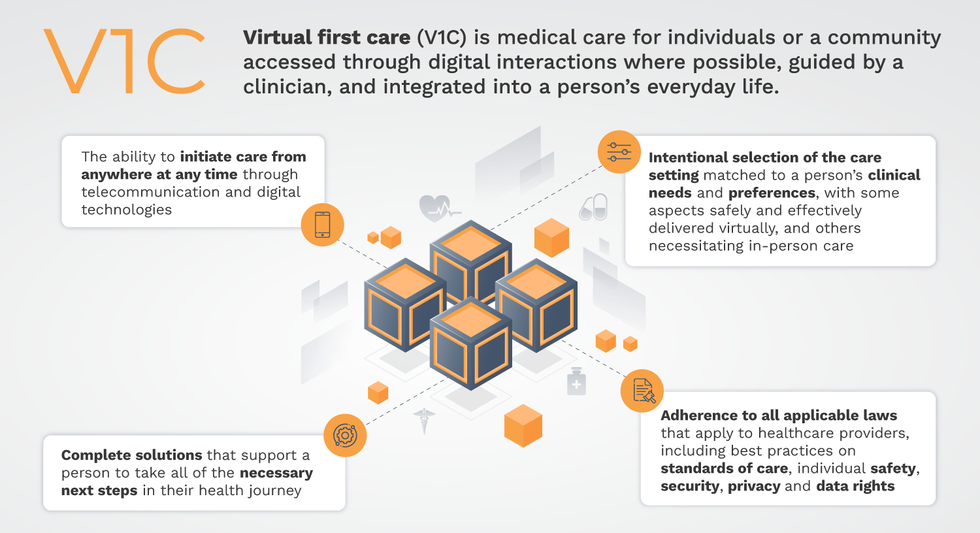
Virtual-first care, or V1C, could increase the quality of healthcare and make it more patient-centric by letting patients combine in-person visits with virtual options such as video for seeing their care providers.
As the pandemic turns endemic, healthcare providers have been eagerly urging patients to return to their offices to enjoy the benefits of in-person care.
But wait.
The last two years have forced all sorts of organizations to be nimble, adaptable and creative in how they work, and this includes healthcare providers’ efforts to maintain continuity of care under the most challenging of conditions. So before we go back to “business as usual,” don’t we owe it to those providers and ourselves to admit that business as usual did not work for most of the people the industry exists to help? If we’re going to embrace yet another period of change – periods that don’t happen often in our complex industry – shouldn’t we first stop and ask ourselves what we’re trying to achieve?
Certainly, COVID has shown that telehealth can be an invaluable tool, particularly for patients in rural and underserved communities that lack access to specialty care. It’s also become clear that many – though not all – healthcare encounters can be effectively conducted from afar. That said, the telehealth tactics that filled the gap during the pandemic were largely stitched together substitutes for existing visit-based workflows: with offices closed, patients scheduled video visits for help managing the side effects of their blood pressure medications or to see their endocrinologist for a quarterly check-in. Anyone whose children slogged through the last year or two of remote learning can tell you that simply virtualizing existing processes doesn’t necessarily improve the experience or the outcomes!
But what if our approach to post-pandemic healthcare came from a patient-driven perspective? We have a fleeting opportunity to advance a care model centered on convenient and equitable access that first prioritizes good outcomes, then selects approaches to care – and locations – tailored to each patient. Using the example of education, imagine how effective it would be if each student, regardless of their school district and aptitude, received such individualized attention.
That’s the idea behind virtual-first care (V1C), a new care model centered on convenient, customized, high-quality care that integrates a full suite of services tailored directly to patients’ clinical needs and preferences. This package includes asynchronous communication such as texting; video and other live virtual modes; and in-person options.
V1C goes beyond what you might think of as standard “telehealth” by using evidence-based protocols and tools that include traditional and digital therapeutics and testing, personalized care plans, dynamic patient monitoring, and team-based approaches to care. This could include spit kits mailed for laboratory tests and complementing clinical care with health coaching. V1C also replaces some in-person exams with ongoing monitoring, using sensors for more ‘whole person’ care.
Amidst all this momentum, we have the opportunity to rethink the goals of healthcare innovation, but that means bringing together key stakeholders to demonstrate that sustainable V1C can redefine healthcare.
Established V1C healthcare providers such as Omada, Headspace, and Heartbeat Health, as well as emerging market entrants like Oshi, Visana, and Wellinks, work with a variety of patients who have complicated long-term conditions such as diabetes, heart failure, gastrointestinal illness, endometriosis, and COPD. V1C is comprehensive in ways that are lacking in digital health and its other predecessors: it has the potential to integrate multiple data streams, incorporate more frequent touches and check-ins over time, and manage a much wider range of chronic health conditions, improving lives and reducing disease burden now and in the future.
Recognizing the pandemic-driven interest in virtual care, significant energy and resources are already flowing fast toward V1C. Some of the world’s largest innovators jumped into V1C early on: Verily, Alphabet’s Life Sciences Company, launched Onduo in 2016 to disrupt the diabetes healthcare market, and is now well positioned to scale its solutions. Major insurers like Aetna and United now offer virtual-first plans to members, responding as organizations expand virtual options for employees. Amidst all this momentum, we have the opportunity to rethink the goals of healthcare innovation, but that means bringing together key stakeholders to demonstrate that sustainable V1C can redefine healthcare.
That was the immediate impetus for IMPACT, a consortium of V1C companies, investors, payers and patients founded last year to ensure access to high-quality, evidence-based V1C. Developed by our team at the Digital Medicine Society (DiMe) in collaboration with the American Telemedicine Association (ATA), IMPACT has begun to explore key issues that include giving patients more integrated experiences when accessing both virtual and brick-and-mortar care.
Digital Medicine Society
V1C is not, nor should it be, virtual-only care. In this new era of hybrid healthcare, success will be defined by how well providers help patients navigate the transitions. How do we smoothly hand a patient off from an onsite primary care physician to, say, a virtual cardiologist? How do we get information from a brick-and-mortar to a digital portal? How do you manage dataflow while still staying HIPAA compliant? There are many complex regulatory implications for these new models, as well as an evolving landscape in terms of privacy, security and interoperability. It will be no small task for groups like IMPACT to determine the best path forward.
None of these factors matter unless the industry can recruit and retain clinicians. Our field is facing an unprecedented workforce crisis. Traditional healthcare is making clinicians miserable, and COVID has only accelerated the trend of overworked, disenchanted healthcare workers leaving in droves. Clinicians want more interactions with patients, and fewer with computer screens – call it “More face time, less FaceTime.” No new model will succeed unless the industry can more efficiently deploy its talent – arguably its most scarce and precious resource. V1C can help with alleviating the increasing burden and frustration borne by individual physicians in today’s status quo.
In healthcare, new technological approaches inevitably provoke no shortage of skepticism. Past lessons from Silicon Valley-driven fixes have led to understandable cynicism. But V1C is a different breed of animal. By building healthcare around the patient, not the clinic, V1C can make healthcare work better for patients, payers and providers. We’re at a fork in the road: we can revert back to a broken sick-care system, or dig in and do the hard work of figuring out how this future-forward healthcare system gets financed, organized and executed. As a field, we must find the courage and summon the energy to embrace this moment, and make it a moment of change.
Podcast: The future of brain health with Percy Griffin
Percy Griffin, director of scientific engagement for the Alzheimer’s Association, joins Leaps.org to discuss the present and future of the fight against dementia.
Today's guest is Percy Griffin, director of scientific engagement for the Alzheimer’s Association, a nonprofit that’s focused on speeding up research, finding better ways to detect Alzheimer’s earlier and other approaches for reducing risk. Percy has a doctorate in molecular cell biology from Washington University, he’s led important research on Alzheimer’s, and you can find the link to his full bio in the show notes, below.
Our topic for this conversation is the present and future of the fight against dementia. Billions of dollars have been spent by the National Institutes of Health and biotechs to research new treatments for Alzheimer's and other forms of dementia, but so far there's been little to show for it. Last year, Aduhelm became the first drug to be approved by the FDA for Alzheimer’s in 20 years, but it's received a raft of bad publicity, with red flags about its effectiveness, side effects and cost.
Meanwhile, 6.5 million Americans have Alzheimer's, and this number could increase to 13 million in 2050. Listen to this conversation if you’re concerned about your own brain health, that of family members getting older, or if you’re just concerned about the future of this country with experts predicting the number people over 65 will increase dramatically in the very near future.
Listen to the Episode
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
4:40 - We talk about the parts of Percy’s life that led to him to concentrate on working in this important area.
6:20 - He defines Alzheimer's and dementia, and discusses the key elements of communicating science.
10:20 - Percy explains why the Alzheimer’s Association has been supportive of Aduhelm, even as others have been critical.
17:58 - We talk about therapeutics under development, which ones to be excited about, and how they could be tailored to a person's own biology.
24:25 - Percy discusses funding and tradeoffs between investing more money into Alzheimer’s research compared to other intractable diseases like cancer, and new opportunities to accelerate progress, such as ARPA-H, President Biden’s proposed agency to speed up health breakthroughs.
27:24 - We talk about the social determinants of brain health. What are the pros/cons of continuing to spend massive sums of money to develop new drugs like Aduhelm versus refocusing on expanding policies to address social determinants - like better education, nutritious food and safe drinking water - that have enabled some groups more than others to enjoy improved cognition late in life.
34:18 - Percy describes his top lifestyle recommendations for protecting your mind.
37:33 - Is napping bad for the brain?
39:39 - Circadian rhythm and Alzheimer's.
42:34 - What tests can people take to check their brain health today, and which biomarkers are we making progress on?
47:25 - Percy highlights important programs run by the Alzheimer’s Association to support advances.
Show links:
** After this episode was recorded, the Centers for Medicare and Medicaid Services affirmed its decision from last June to limit coverage of Aduhelm. More here.
- Percy Griffin's bio: https://www.alz.org/manh/events/alztalks/upcoming-...
- The Alzheimer's Association's Part the Cloud program: https://alz.org/partthecloud/about-us.asp
- The paradox of dementia rates decreasing: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7455342/
- The argument for focusing more resources on improving institutions and social processes for brain health: https://www.statnews.com/2021/09/23/the-brain-heal...
- Recent research on napping: https://www.ocregister.com/2022/03/25/alzheimers-s...
- The Alzheimer's Association helpline: https://www.alz.org/help-support/resources/helpline
- ALZConnected, a free online community for people affected by dementia https://www.alzconnected.org/
- TrialMatch for people with dementia and healthy volunteers to find clinical trials for Alzheimer's and other dementia: https://www.alz.org/alzheimers-dementia/research_p...

