People With This Rare Disease Can Barely Eat Protein. Biotechnology May Change That.

The Brown family at the Grand Tetons (2019). Clockwise from left, Christine, Kevin, Keagan, Connor, and Kellen.
Imagine that the protein in bread, eggs, steak, even beans is not the foundation for a healthy diet, but a poison to your brain. That is the reality for people living with Phenylketonuria, or PKU. This cluster of rare genetic variations affects the ability to digest phenylalanine (Phe), one of the chemical building blocks of protein. The toxins can build up in the brain causing severe mental retardation.
Can a probiotic help digest the troublesome proteins before they can enter the bloodstream and travel to the brain? A Boston area biotech start up, Synlogic, believes it can. Their starting point is an E. coli bacterium that has been used as a probiotic for more than a century. The company then screened thousands of gene variants to identify ones that produced enzymes most efficient at slicing and dicing the target proteins and optimized them further through directed evolution. The results have been encouraging.
But Christine Brown knew none of this when the hospital called saying that standard newborn screening of her son Connor had come back positive for PKU. It was urgent that they visit a special metabolic clinic the next day, which was about a three-hour drive away.
“I was told not to go on the Internet,” Christine recalls, “So when somebody tells you not to go on the Internet, what do you do? Even back in 2005, right.” What she saw were the worst examples of retardation, which was a common outcome from PKU before newborn screening became routine. “We were just in complete shell shock, our whole world just kind of shattered and went into a tail spin.”
“I remember feeding him the night before our clinic visit and almost feeling like I was feeding him poison because I knew that breast milk must have protein in it,” she says.
“Some of my first memories are of asking, ‘Mommy, can I eat this? There were yes foods and no foods.'"
Over the next few days the dedicated staff of the metabolic clinic at the Waisman Center at the University of Wisconsin Madison began to walk she and husband Kevin back from that nightmare. They learned that a simple blood test to screen newborns had been developed in the early 1960s to detect PKU and that the condition could be managed with stringent food restrictions and vigilant monitoring of Phe levels.
Everything in Your Mouth Counts
PKU can be successfully managed with a severely restricted diet. That simple statement is factually true, but practically impossible to follow, as it requires slashing protein consumption by about 90 percent. To compensate for the missing protein, several times a day PKU patients take a medical formula – commonly referred to simply as formula – containing forms of proteins that are digestible to their bodies. Several manufacturers now add vitamins and minerals and offer a variety of formats and tastes to make it more consumer friendly, but that wasn't always the case.
“When I was a kid, it tasted horrible, was the consistency of house paint. I didn't think about it, I just drank it. I didn't like it but you get used to it after a while,” recalls Jeff Wolf, the twang of Appalachia still strong in his voice. Now age 50, he grew up in Ashland, Kentucky and was part of the first wave of persons with PKU who were identified at birth as newborn screening was rolled out across the US. He says the options of taste and consistency have improved tremendously over the years.
Some people with PKU are restricted to as little as 8 grams of protein a day from food. That's a handful of almonds or a single hard-boiled egg; a skimpy 4-ounce hamburger and slice of cheese adds up to half of their weekly protein ration. Anything above that daily allowance is more than the body can handle and toxic levels of Phe begin to accumulate in the brain.
“Some of my first memories are of asking, ‘Mommy, can I eat this? There were yes foods and no foods,’” recalls Les Clark. He has never eaten a hamburger, steak, or ribs, practically a sacrilege for someone raised in Stanton, a small town in northeastern Nebraska, a state where the number of cattle and hogs are several-fold those of people.
His grandmother learned how to make low protein bread, but it looked and tasted different. His mom struggled making birthday cakes. “I learned some bad words at a very young age” as mom struggled applying icing that would pull the cake apart or a slice would collapse into a heap of crumbs, Les recalls.

Les Clark with a birthday cake.
Courtesy Clark
Controlling the diet “is not so bad when you are a baby” because that's all you know, says Jerry Vockley, Director of the Center for Rare Disease Therapy at Children's Hospital of Pittsburgh. “But after a while, as you get older and you start tasting other things and you say, Well, gee, this stuff tastes way better than what you're giving me. What's the deal? It becomes harder to maintain the diet.”
First is the lure of forbidden foods as children venture into the community away from the watchful eyes of parents. The support system weakens further when they leave home for college or work. “Pizza was mighty tasty,” Wolf' says of his first slice.
Vockley estimates that about 90 percent of adults with PKU are off of treatment. Moving might mean finding a new metabolic clinic that treats PKU. A lapse in insurance coverage can be a factor. Finally there is plain fatigue from multiple daily dosing of barely tolerable formula, monitoring protein intake, and simply being different in terms of food restrictions. Most people want to fit in and not be defined by their medical condition.
Jeff Wolf was one of those who dropped out in his twenties and thirties. He stopped going to clinic, monitoring his Phe levels, and counting protein. But the earlier experience of living with PKU never completely left the back of his mind; he listened to his body whenever eating too much protein left him with the “fuzzy brain" of a protein hangover. About a decade ago he reconnected with a metabolic clinic, began taking formula and watching his protein intake. He still may go over his allotment for a single day but he tries to compensate on subsequent days so that his Phe levels come back into balance.

Jeff Wolf on a boat.
Courtesy Wolf
One of the trickiest parts of trying to manage phenylalanine intake is the artificial sweetener aspartame. The chemical is ubiquitous in diet and lite foods and drinks. Gum too, you don't even have to swallow to receive a toxic dose of Phe. Most PKUers say it is easier to simply avoid these products entirely rather than try to count their Phe content.
Treatments
Most rare diseases have no treatment. There are two drugs for PKU that provide some benefit to some portion of patients but those drugs often have their own burdens.
KUVAN® (sapropterin dihydrochloride) is a pill or powder that helps correct a protein folding error so that food proteins can be digested. It is approved for most types of PKU in adults and children one month and older, and often is used along with a protein-restricted diet.
“The problem is that it doesn't work for every [patient's genetic] mutation, and there are hundreds of mutations that have been identified with PKU. Two to three percent of patients will have a very dramatic response and if you're one of those small number of patients, it's great,” says Vockley. “If you have one of the other mutation, chances are pretty good you still are going to end up on a restricted diet.”
PALYNZIQ® (pegvaliase-pqpz) “has the potential to lower the Phe to normal levels, it's a real breakthrough in the field,” says Vockley. “But is a very hard drug to use. Most folks have to take either one or two 2ml injections a day of something that is basically a gel, and some individuals have to take three.”
Many PKUers have reactions at the site of the injection and some develop anaphylaxis, a severe potentially life-threatening allergic reaction that can happen within seconds and can occur at any time, even after long term use. Many patients using Palynziq carry an EpiPen, a self-injection devise containing a form of adrenaline that can reverse some of the symptoms of anaphylaxis.
Then there is the cost. With the Kuvan dosing for an adult, “you're talking between $100,000 and $200,000 a year. And Palynziq is three times that,” says Vockley. Insurance coverage through a private plan or a state program is essential. Some state programs provide generous coverage while others are skimpy. Most large insurance company plans cover the drugs, sometimes with significant copays, but companies that are self-insured are under no legal obligation to provide coverage.
Les Clark found that out the hard way when the company he worked for was sold. The new owner was self-insured and declined to continue covering his drugs. Almost immediately he was out of pocket an additional $1500 a month for formula, and that was with a substantial discount through the manufacturer's patient support program. He says, “If you don't have an insurance policy that will cover the formula, it's completely unaffordable.” He quickly began to look for a new job.
Hope
It's easy to see why PKUers are eager for advances that will make managing their condition more effect, easier, and perhaps more affordable. Synlogic's efforts have drawn their attention and raised hopes.
Just before Thanksgiving Jerry Vockley presented the latest data to a metabolism conference meeting in Australia. There were only 8 patients in this group of a phase 2 trial using the original version of the company's lead E. coli product, SYNB1618, but they were intensely studied. Each was given the probiotic and then a challenge meal. Vockley saw a 40% reduction in Phe absorption and later a 20% reduction in mean fasting Phe levels in the blood. The product was easy to use and tolerate.
The company also presented early results for SYNB1934, a follow on version that further genetically tweaked the E. coli to roughly double the capacity to chop up the target proteins. Synlogic is recruiting patients for studies to determine the best dosing, which they are planning for next year.
“It's an exciting approach,” says Lex Cowsert, Director of Research Development at the National PKU Alliance, a nonprofit that supports the patient, family, and research communities involved with PKU. “Every patient is different, every patient has a different tolerance for the type of therapy that they are willing to pursue,” and if it pans out, it will be a welcome addition, either alone or in combination with other approaches, to living with PKU.
Author's Note: Reporting this story was made possible by generous support from the National Press Foundation and the Fondation Ipsen. Thanks to the people who so generously shared their time and stories in speaking with me.
Dr. May Edward Chinn, Kizzmekia Corbett, PhD., and Alice Ball, among others, have been behind some of the most important scientific work of the last century.
If you look back on the last century of scientific achievements, you might notice that most of the scientists we celebrate are overwhelmingly white, while scientists of color take a backseat. Since the Nobel Prize was introduced in 1901, for example, no black scientists have landed this prestigious award.
The work of black women scientists has gone unrecognized in particular. Their work uncredited and often stolen, black women have nevertheless contributed to some of the most important advancements of the last 100 years, from the polio vaccine to GPS.
Here are five black women who have changed science forever.
Dr. May Edward Chinn
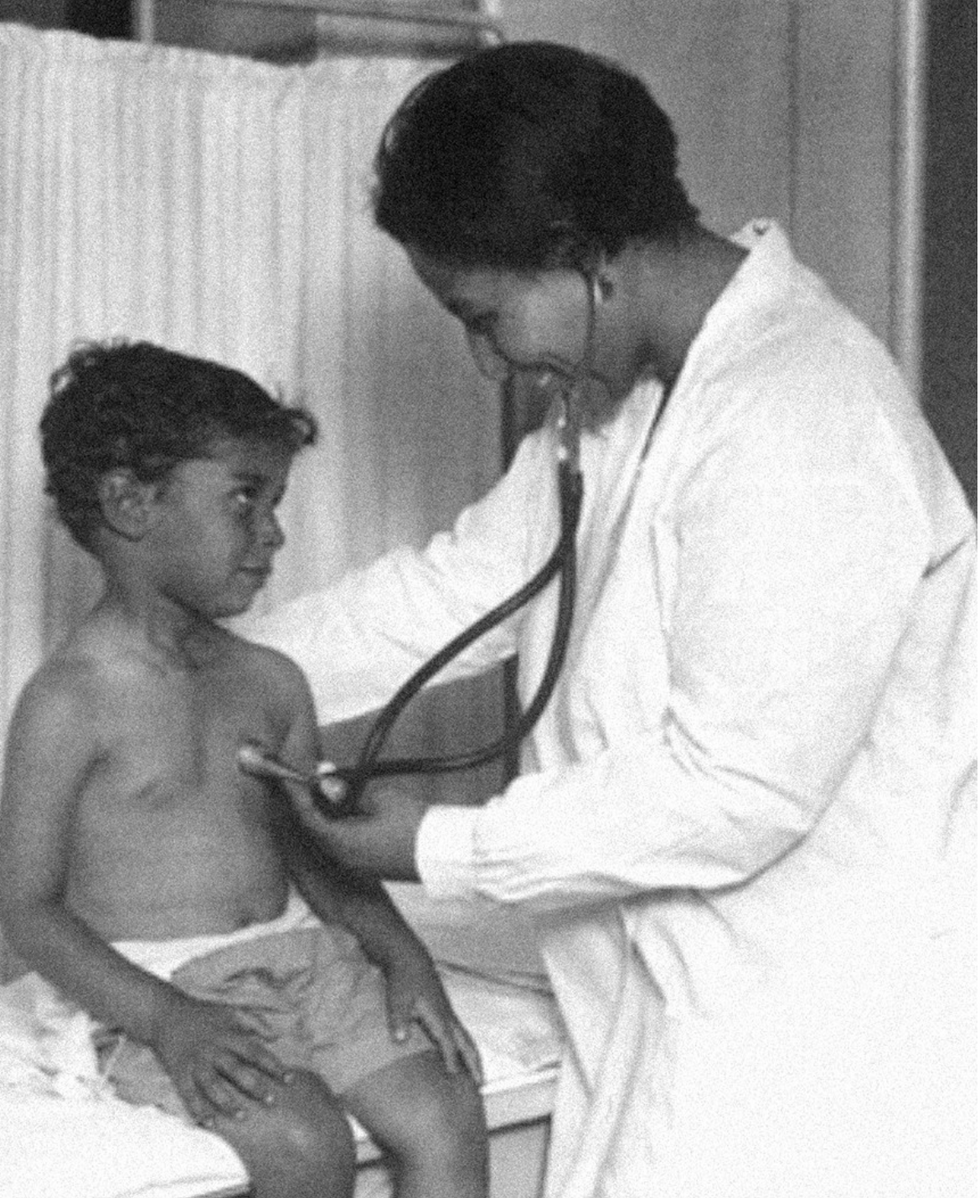
Dr. May Edward Chinn practicing medicine in Harlem
George B. Davis, PhD.
Chinn was born to poor parents in New York City just before the start of the 20th century. Although she showed great promise as a pianist, playing with the legendary musician Paul Robeson throughout the 1920s, she decided to study medicine instead. Chinn, like other black doctors of the time, were barred from studying or practicing in New York hospitals. So Chinn formed a private practice and made house calls, sometimes operating in patients’ living rooms, using an ironing board as a makeshift operating table.
Chinn worked among the city’s poor, and in doing this, started to notice her patients had late-stage cancers that often had gone undetected or untreated for years. To learn more about cancer and its prevention, Chinn begged information off white doctors who were willing to share with her, and even accompanied her patients to other clinic appointments in the city, claiming to be the family physician. Chinn took this information and integrated it into her own practice, creating guidelines for early cancer detection that were revolutionary at the time—for instance, checking patient health histories, checking family histories, performing routine pap smears, and screening patients for cancer even before they showed symptoms. For years, Chinn was the only black female doctor working in Harlem, and she continued to work closely with the poor and advocate for early cancer screenings until she retired at age 81.
Alice Ball

Pictorial Press Ltd/Alamy
Alice Ball was a chemist best known for her groundbreaking work on the development of the “Ball Method,” the first successful treatment for those suffering from leprosy during the early 20th century.
In 1916, while she was an undergraduate student at the University of Hawaii, Ball studied the effects of Chaulmoogra oil in treating leprosy. This oil was a well-established therapy in Asian countries, but it had such a foul taste and led to such unpleasant side effects that many patients refused to take it.
So Ball developed a method to isolate and extract the active compounds from Chaulmoogra oil to create an injectable medicine. This marked a significant breakthrough in leprosy treatment and became the standard of care for several decades afterward.
Unfortunately, Ball died before she could publish her results, and credit for this discovery was given to another scientist. One of her colleagues, however, was able to properly credit her in a publication in 1922.
Henrietta Lacks

onathan Newton/The Washington Post/Getty
The person who arguably contributed the most to scientific research in the last century, surprisingly, wasn’t even a scientist. Henrietta Lacks was a tobacco farmer and mother of five children who lived in Maryland during the 1940s. In 1951, Lacks visited Johns Hopkins Hospital where doctors found a cancerous tumor on her cervix. Before treating the tumor, the doctor who examined Lacks clipped two small samples of tissue from Lacks’ cervix without her knowledge or consent—something unthinkable today thanks to informed consent practices, but commonplace back then.
As Lacks underwent treatment for her cancer, her tissue samples made their way to the desk of George Otto Gey, a cancer researcher at Johns Hopkins. He noticed that unlike the other cell cultures that came into his lab, Lacks’ cells grew and multiplied instead of dying out. Lacks’ cells were “immortal,” meaning that because of a genetic defect, they were able to reproduce indefinitely as long as certain conditions were kept stable inside the lab.
Gey started shipping Lacks’ cells to other researchers across the globe, and scientists were thrilled to have an unlimited amount of sturdy human cells with which to experiment. Long after Lacks died of cervical cancer in 1951, her cells continued to multiply and scientists continued to use them to develop cancer treatments, to learn more about HIV/AIDS, to pioneer fertility treatments like in vitro fertilization, and to develop the polio vaccine. To this day, Lacks’ cells have saved an estimated 10 million lives, and her family is beginning to get the compensation and recognition that Henrietta deserved.
Dr. Gladys West

Andre West
Gladys West was a mathematician who helped invent something nearly everyone uses today. West started her career in the 1950s at the Naval Surface Warfare Center Dahlgren Division in Virginia, and took data from satellites to create a mathematical model of the Earth’s shape and gravitational field. This important work would lay the groundwork for the technology that would later become the Global Positioning System, or GPS. West’s work was not widely recognized until she was honored by the US Air Force in 2018.
Dr. Kizzmekia "Kizzy" Corbett

TIME Magazine
At just 35 years old, immunologist Kizzmekia “Kizzy” Corbett has already made history. A viral immunologist by training, Corbett studied coronaviruses at the National Institutes of Health (NIH) and researched possible vaccines for coronaviruses such as SARS (Severe Acute Respiratory Syndrome) and MERS (Middle East Respiratory Syndrome).
At the start of the COVID pandemic, Corbett and her team at the NIH partnered with pharmaceutical giant Moderna to develop an mRNA-based vaccine against the virus. Corbett’s previous work with mRNA and coronaviruses was vital in developing the vaccine, which became one of the first to be authorized for emergency use in the United States. The vaccine, along with others, is responsible for saving an estimated 14 million lives.On today’s episode of Making Sense of Science, I’m honored to be joined by Dr. Paul Song, a physician, oncologist, progressive activist and biotech chief medical officer. Through his company, NKGen Biotech, Dr. Song is leveraging the power of patients’ own immune systems by supercharging the body’s natural killer cells to make new treatments for Alzheimer’s and cancer.
Whereas other treatments for Alzheimer’s focus directly on reducing the build-up of proteins in the brain such as amyloid and tau in patients will mild cognitive impairment, NKGen is seeking to help patients that much of the rest of the medical community has written off as hopeless cases, those with late stage Alzheimer’s. And in small studies, NKGen has shown remarkable results, even improvement in the symptoms of people with these very progressed forms of Alzheimer’s, above and beyond slowing down the disease.
In the realm of cancer, Dr. Song is similarly setting his sights on another group of patients for whom treatment options are few and far between: people with solid tumors. Whereas some gradual progress has been made in treating blood cancers such as certain leukemias in past few decades, solid tumors have been even more of a challenge. But Dr. Song’s approach of using natural killer cells to treat solid tumors is promising. You may have heard of CAR-T, which uses genetic engineering to introduce cells into the body that have a particular function to help treat a disease. NKGen focuses on other means to enhance the 40 plus receptors of natural killer cells, making them more receptive and sensitive to picking out cancer cells.
Paul Y. Song, MD is currently CEO and Vice Chairman of NKGen Biotech. Dr. Song’s last clinical role was Asst. Professor at the Samuel Oschin Cancer Center at Cedars Sinai Medical Center.
Dr. Song served as the very first visiting fellow on healthcare policy in the California Department of Insurance in 2013. He is currently on the advisory board of the Pritzker School of Molecular Engineering at the University of Chicago and a board member of Mercy Corps, The Center for Health and Democracy, and Gideon’s Promise.
Dr. Song graduated with honors from the University of Chicago and received his MD from George Washington University. He completed his residency in radiation oncology at the University of Chicago where he served as Chief Resident and did a brachytherapy fellowship at the Institute Gustave Roussy in Villejuif, France. He was also awarded an ASTRO research fellowship in 1995 for his research in radiation inducible gene therapy.
With Dr. Song’s leadership, NKGen Biotech’s work on natural killer cells represents cutting-edge science leading to key findings and important pieces of the puzzle for treating two of humanity’s most intractable diseases.
Show links
- Paul Song LinkedIn
- NKGen Biotech on Twitter - @NKGenBiotech
- NKGen Website: https://nkgenbiotech.com/
- NKGen appoints Paul Song
- Patient Story: https://pix11.com/news/local-news/long-island/promising-new-treatment-for-advanced-alzheimers-patients/
- FDA Clearance: https://nkgenbiotech.com/nkgen-biotech-receives-ind-clearance-from-fda-for-snk02-allogeneic-natural-killer-cell-therapy-for-solid-tumors/Q3 earnings data: https://www.nasdaq.com/press-release/nkgen-biotech-inc.-reports-third-quarter-2023-financial-results-and-business