Powerful New Technologies Are Speeding the Development of a Coronavirus Vaccine
Dr. Adalja is focused on emerging infectious disease, pandemic preparedness, and biosecurity. He has served on US government panels tasked with developing guidelines for the treatment of plague, botulism, and anthrax in mass casualty settings and the system of care for infectious disease emergencies, and as an external advisor to the New York City Health and Hospital Emergency Management Highly Infectious Disease training program, as well as on a FEMA working group on nuclear disaster recovery. Dr. Adalja is an Associate Editor of the journal Health Security. He was a coeditor of the volume Global Catastrophic Biological Risks, a contributing author for the Handbook of Bioterrorism and Disaster Medicine, the Emergency Medicine CorePendium, Clinical Microbiology Made Ridiculously Simple, UpToDate's section on biological terrorism, and a NATO volume on bioterrorism. He has also published in such journals as the New England Journal of Medicine, the Journal of Infectious Diseases, Clinical Infectious Diseases, Emerging Infectious Diseases, and the Annals of Emergency Medicine. He is a board-certified physician in internal medicine, emergency medicine, infectious diseases, and critical care medicine. Follow him on Twitter: @AmeshAA
A lab tech handling a sample of the novel coronavirus.
One of the main factors that will influence the ultimate trajectory of the novel coronavirus pandemic will be the availability of a vaccine.
Vaccine development has traditionally been a process measured in years and even decades.
Vaccines are incontrovertibly the best means to control infectious diseases and there are no human vaccines against any of the (now) 7 known human coronaviruses. As soon as the gravity of this outbreak was recognized, several companies, along with governmental and non-governmental partners, have embarked on a rapid development program to develop a vaccine targeted at this virus.
Vaccine development has traditionally been a process measured in years and even decades as scientists tinker with a pathogen trying to weaken or dissemble it to render it capable of creating an effective immune response with acceptable levels of side effects. However, in 2020, powerful new vaccine technologies are available to augment traditional vaccine development and are responsible for the rapid delivery of a vaccine candidate for the start of clinical trials.
Vaccine Platforms: A Game-Changing Technology
The new technologies that are being harnessed are what are known as vaccine platform technologies. Vaccine platforms, as my colleagues and I wrote in a report assessing their promise, offer a means to use the same building blocks to make more than one vaccine. To slightly oversimply, a vaccine platform confers the ability to switch out the pathogen being targeted very rapidly, akin to changing a video game cartridge. Indeed, the recently FDA-licensed Ebola vaccine uses another virus as a platform with the requisite Ebola protein inserted.
Because of this rapid availability to utilize platforms for a variety of different targets, the initial development process can be significantly shortened. This is especially true for vaccines utilizing the genetic material of the target alone. These DNA and RNA vaccines basically can be "printed" once the genetic sequence of the target is known.
An RNA vaccine is the approach being used by the Cambridge-based biotech company Moderna – which took just 42 days to produce an experimental vaccine candidate. Clinical testing is expected to begin next month on 45 healthy volunteers.
Another biotech, the Pennsylvania-based Inovio, is using a DNA approach. In essence, such vaccines involve the genetic material being injected and translated into a viral protein by human cells, which then prompt the immune system to make antibodies.
There are other approaches as well. One company, the Maryland-based Novavax, will use nanoparticles, while another is attempting to adapt an orally administered avian coronavirus vaccine and Johnson & Johnson is using different virus platforms to deliver coronavirus proteins (similar to their experimental Ebola vaccine).
At this stage, it is important for all approaches to be on the table in the hope that at least one makes it through clinical trials. There also may be a need for different types of vaccines for different populations.
Vaccines Will Still Take Time
Despite the quick development time made possible by the use of vaccine platforms, clinical testing for safety, efficacy, and dosing schedules will still take months to complete. After this process, the vaccine will need to be mass produced in large quantities to vaccinate, basically, the world. So, for all intents and purposes, we cannot expect to see an approved vaccine for at least a year or maybe longer if everything does not go perfectly well in clinical trials.
Vaccine platform technologies offer a bright ray of hope in the bleak shadow of the pandemic.
Once a vaccine is available, it will likely appear in batches to be distributed to those at highest risk for severe disease, such as the elderly and those with underlying conditions, as well as healthcare workers, first. At this time, it appears children are less likely to experience severe illness and they may not be the first targets for the vaccine but, if this virus is with us (as is predicted), coronavirus vaccination could become part of routine childhood vaccinations.
Changing Pandemic Trajectory
Vaccination will not come fast enough to impact the initial wave of the novel virus which may continue until summer approaches in temperate climates. However, it will be a crucial tool to blunt the impact of a future appearance in the following respiratory virus season. This reappearance is all but assured as this virus has adeptly established itself in human populations and is behaving like the community-acquired coronavirus that it is.
A Glimmer of Hope
When looking at the trajectory of the virus, it can appear, thus far, that no public health effort has made a substantial impact on the spread of the virus. However, that trajectory will change with the advent of an efficacious vaccine. Such a vaccine, especially if conferring protection against other human coronaviruses, may result in coronaviruses being taken off the table of biological threats altogether in the future.
Vaccine platform technologies offer a bright ray of hope in the bleak shadow of the pandemic and, if successful, will change the way the world approaches future pandemic threats with more rapid deployment of platform-based vaccines.
Dr. Adalja is focused on emerging infectious disease, pandemic preparedness, and biosecurity. He has served on US government panels tasked with developing guidelines for the treatment of plague, botulism, and anthrax in mass casualty settings and the system of care for infectious disease emergencies, and as an external advisor to the New York City Health and Hospital Emergency Management Highly Infectious Disease training program, as well as on a FEMA working group on nuclear disaster recovery. Dr. Adalja is an Associate Editor of the journal Health Security. He was a coeditor of the volume Global Catastrophic Biological Risks, a contributing author for the Handbook of Bioterrorism and Disaster Medicine, the Emergency Medicine CorePendium, Clinical Microbiology Made Ridiculously Simple, UpToDate's section on biological terrorism, and a NATO volume on bioterrorism. He has also published in such journals as the New England Journal of Medicine, the Journal of Infectious Diseases, Clinical Infectious Diseases, Emerging Infectious Diseases, and the Annals of Emergency Medicine. He is a board-certified physician in internal medicine, emergency medicine, infectious diseases, and critical care medicine. Follow him on Twitter: @AmeshAA
Thanks to safety cautions from the COVID-19 pandemic, a strain of influenza has been completely eliminated.
If you were one of the millions who masked up, washed your hands thoroughly and socially distanced, pat yourself on the back—you may have helped change the course of human history.
Scientists say that thanks to these safety precautions, which were introduced in early 2020 as a way to stop transmission of the novel COVID-19 virus, a strain of influenza has been completely eliminated. This marks the first time in human history that a virus has been wiped out through non-pharmaceutical interventions, such as vaccines.
The flu shot, explained
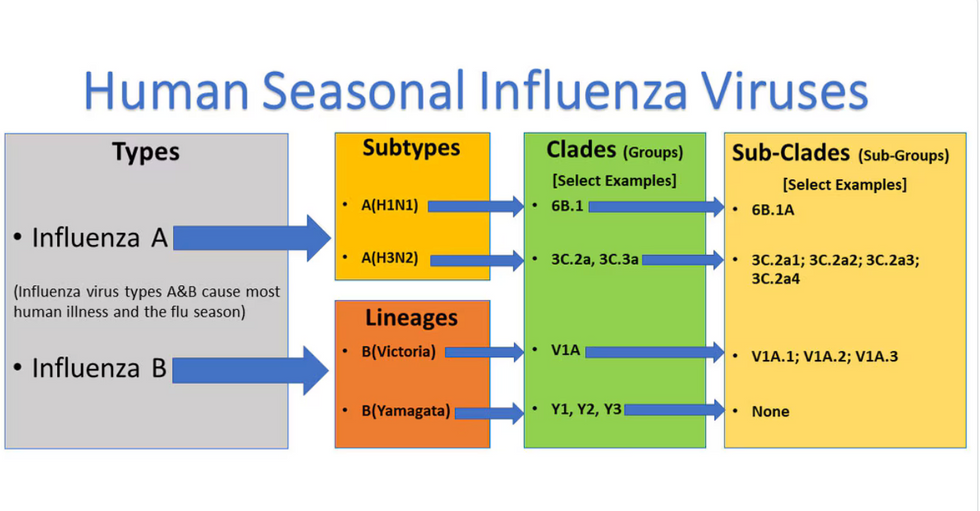
Influenza viruses type A and B are responsible for the majority of human illnesses and the flu season.
Centers for Disease Control
For more than a decade, flu shots have protected against two types of the influenza virus–type A and type B. While there are four different strains of influenza in existence (A, B, C, and D), only strains A, B, and C are capable of infecting humans, and only A and B cause pandemics. In other words, if you catch the flu during flu season, you’re most likely sick with flu type A or B.
Flu vaccines contain inactivated—or dead—influenza virus. These inactivated viruses can’t cause sickness in humans, but when administered as part of a vaccine, they teach a person’s immune system to recognize and kill those viruses when they’re encountered in the wild.
Each spring, a panel of experts gives a recommendation to the US Food and Drug Administration on which strains of each flu type to include in that year’s flu vaccine, depending on what surveillance data says is circulating and what they believe is likely to cause the most illness during the upcoming flu season. For the past decade, Americans have had access to vaccines that provide protection against two strains of influenza A and two lineages of influenza B, known as the Victoria lineage and the Yamagata lineage. But this year, the seasonal flu shot won’t include the Yamagata strain, because the Yamagata strain is no longer circulating among humans.
How Yamagata Disappeared

Flu surveillance data from the Global Initiative on Sharing All Influenza Data (GISAID) shows that the Yamagata lineage of flu type B has not been sequenced since April 2020.
Nature
Experts believe that the Yamagata lineage had already been in decline before the pandemic hit, likely because the strain was naturally less capable of infecting large numbers of people compared to the other strains. When the COVID-19 pandemic hit, the resulting safety precautions such as social distancing, isolating, hand-washing, and masking were enough to drive the virus into extinction completely.
Because the strain hasn’t been circulating since 2020, the FDA elected to remove the Yamagata strain from the seasonal flu vaccine. This will mark the first time since 2012 that the annual flu shot will be trivalent (three-component) rather than quadrivalent (four-component).
Should I still get the flu shot?
The flu shot will protect against fewer strains this year—but that doesn’t mean we should skip it. Influenza places a substantial health burden on the United States every year, responsible for hundreds of thousands of hospitalizations and tens of thousands of deaths. The flu shot has been shown to prevent millions of illnesses each year (more than six million during the 2022-2023 season). And while it’s still possible to catch the flu after getting the flu shot, studies show that people are far less likely to be hospitalized or die when they’re vaccinated.
Another unexpected benefit of dropping the Yamagata strain from the seasonal vaccine? This will possibly make production of the flu vaccine faster, and enable manufacturers to make more vaccines, helping countries who have a flu vaccine shortage and potentially saving millions more lives.
After his grandmother’s dementia diagnosis, one man invented a snack to keep her healthy and hydrated.
Founder Lewis Hornby and his grandmother Pat, sampling Jelly Drops—an edible gummy containing water and life-saving electrolytes.
On a visit to his grandmother’s nursing home in 2016, college student Lewis Hornby made a shocking discovery: Dehydration is a common (and dangerous) problem among seniors—especially those that are diagnosed with dementia.
Hornby’s grandmother, Pat, had always had difficulty keeping up her water intake as she got older, a common issue with seniors. As we age, our body composition changes, and we naturally hold less water than younger adults or children, so it’s easier to become dehydrated quickly if those fluids aren’t replenished. What’s more, our thirst signals diminish naturally as we age as well—meaning our body is not as good as it once was in letting us know that we need to rehydrate. This often creates a perfect storm that commonly leads to dehydration. In Pat’s case, her dehydration was so severe she nearly died.
When Lewis Hornby visited his grandmother at her nursing home afterward, he learned that dehydration especially affects people with dementia, as they often don’t feel thirst cues at all, or may not recognize how to use cups correctly. But while dementia patients often don’t remember to drink water, it seemed to Hornby that they had less problem remembering to eat, particularly candy.

Hornby wanted to create a solution for elderly people who struggled keeping their fluid intake up. He spent the next eighteen months researching and designing a solution and securing funding for his project. In 2019, Hornby won a sizable grant from the Alzheimer’s Society, a UK-based care and research charity for people with dementia and their caregivers. Together, through the charity’s Accelerator Program, they created a bite-sized, sugar-free, edible jelly drop that looked and tasted like candy. The candy, called Jelly Drops, contained 95% water and electrolytes—important minerals that are often lost during dehydration. The final product launched in 2020—and was an immediate success. The drops were able to provide extra hydration to the elderly, as well as help keep dementia patients safe, since dehydration commonly leads to confusion, hospitalization, and sometimes even death.
Not only did Jelly Drops quickly become a favorite snack among dementia patients in the UK, but they were able to provide an additional boost of hydration to hospital workers during the pandemic. In NHS coronavirus hospital wards, patients infected with the virus were regularly given Jelly Drops to keep their fluid levels normal—and staff members snacked on them as well, since long shifts and personal protective equipment (PPE) they were required to wear often left them feeling parched.
In April 2022, Jelly Drops launched in the United States. The company continues to donate 1% of its profits to help fund Alzheimer’s research.

