Meet the Psychologist Using Psychedelics to Treat Racial Trauma

Monnica Williams was stuck. The veteran psychologist wanted to conduct a study using psychedelics, but her university told her they didn't have the expertise to evaluate it via an institutional review board, which is responsible for providing ethical and regulatory oversight for research that involves human participants. Instead, they directed her to a hospital, whose reviewers turned it down, citing research of a banned substance as unethical.
"I said, 'We're not using illegal psilocybin, we're going through Health Canada,'" Williams said. Psilocybin was banned in Canada in 1974, but can now be obtained with an exemption from Health Canada, the federal government's health policy department. After learning this, the hospital review board told Williams they couldn't review her proposal because she's not affiliated with the hospital, after all.
It's all part of balancing bureaucracy with research goals for Williams, a leading expert on racial trauma and psychedelic medicine, as well as obsessive compulsive disorder (OCD), at the University of Ottawa. She's exploring the use of hallucinogenic substances like MDMA and psilocybin — commonly known as ecstasy and magic mushrooms, respectively — to help people of color address the psychological impacts of systemic racism. A prolific researcher, Williams also works as an expert witness, offering clinical evaluations for racial trauma cases.
Scientists have long known that psychedelics produce an altered state of consciousness and openness to new perspectives. For people with mental health conditions who haven't benefited from traditional therapy, psychedelics may be able to help them discover what's causing their pain or trauma, including racial trauma—the mental and emotional injury spurred by racial bias.
"Using psychedelics can not only bring these pain points to the surface for healing, but can reduce the anxiety or response to these memories and allow them to speak openly about them without the pain they bring," Williams says. Her research harnesses the potential of psychedelics to increase neuroplasticity, which includes the brain's ability to build new pathways.
"People of color are dealing with racism all the time, in large and small ways, and even dealing with racism in healthcare, even dealing with racism in therapy."
But she says therapists of color aren't automatically equipped to treat racial trauma. First, she notes, people of color are "vastly underrepresented in the mental health workforce." This is doubly true in psychedelic-assisted psychotherapy, in which a person is guided through a psychedelic session by a therapist or team of therapists, then processes the experience in subsequent therapy sessions.
"On top of that, the therapists of color are getting the same training that the white therapists are getting, so it's not even really guaranteed that they're going to be any better at helping a person that may have racial trauma emerging as part of their experience," she says.
In her own training to become a clinical psychologist at the University of Virginia, Williams says she was taught "how to be a great psychologist for white people." Yet even people of color, she argues, need specialized training to work with marginalized groups, particularly when it comes to MDMA, psilocybin and other psychedelics. Because these drugs can lower natural psychological defense mechanisms, Williams says, it's important for providers to be specially trained.
"People of color are dealing with racism all the time, in large and small ways, and even dealing with racism in healthcare, even dealing with racism in therapy. So [they] generally develop a lot of defenses and coping strategies to ward off racism so that they can function." she says. This is particularly true with psychedelic-assisted psychotherapy: "One possibility is that you're going to be stripped of your defenses, you're going to be vulnerable. And so you have to work with a therapist who is going to understand that and not enact more racism in their work with you."
Williams has struggled to find funding and institutional approval for research involving psychedelics, or funding for investigations into racial trauma or the impacts of conditions like OCD and post-traumatic stress disorder (PTSD) in people of color. With the bulk of her work focusing on OCD, she hoped to focus on people of color, but found there was little funding for that type of research. In 2020, that started to change as structural racism garnered more media attention.
After the killing of George Floyd, a 46-year-old Black man, by a white police officer in May 2020, Williams was flooded with media requests. "Usually, when something like that happens, I get contacted a lot for a couple of weeks, and it dies off. But after George Floyd, it just never did."
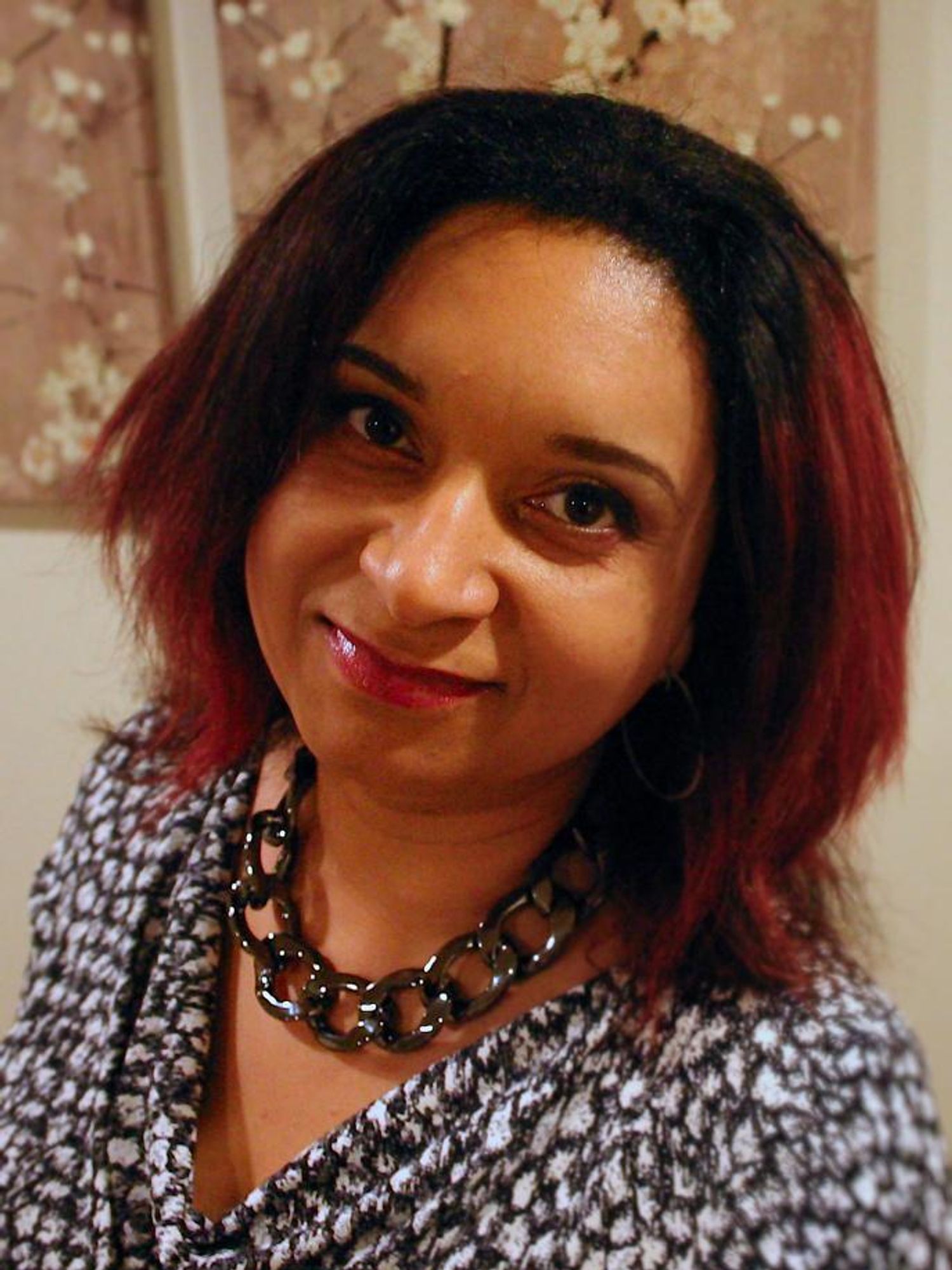
Monnica Williams, clinical psychologist at the University of Ottawa
Williams was no stranger to the questions that soon blazed across headlines: How can we mitigate microaggressions? How do race and ethnicity impact mental health? What terms should we use to discuss racial issues? What constitutes an ally, and why aren't there more of them? Why aren't there more people of color in academia, and so many other fields?
Now, she's hoping that the increased attention on racial justice will mean more acceptance for the kind of research she's doing.
In fact, Williams herself has used psychedelics in order to gain a better understanding of how to use them to treat racial trauma. In a study published in January, she and two other Black female psychotherapists took MDMA in a supervised setting, guided by a team of mental health practitioners who helped them process issues that came up as the session progressed. Williams, who was also the study's lead author, found that participants' experiences centered around processing and finding release from racial identities, and, in one case, of simply feeling wholly human without the burden of racial identity for the first time.
The purpose of the study was twofold: to understand how Black women react to psychedelics and to provide safe, firsthand, psychedelic experiences to Black mental health practitioners. One of the other study participants has since gone on to offer psychedelic-assisted psychotherapy to her own patients.
Psychedelic research, and psilocybin in particular, has become a hot topic of late, particularly after Oregon became the first state to legalize it for therapeutic use last November. A survey-based, observational study with 313 participants, published in 2020, paved the way for Williams' more recent MDMA experiments by describing improvements in depression, anxiety and racial trauma among people of color who had used LSD, psilocybin or MDMA in a non-research setting.
Williams and her team included only respondents who reported a moderate to strong psychoactive effect of past psychedelic consumption and believed these experiences provided "relief from the challenging effects of ethnic discrimination." Participants reported a memorable psychedelic experience as well as its acute and lasting effects, completing assessments of psychological insight, mystical experience and emotional challenges experienced during psychedelic experience, then describing their mental health — including depression, anxiety and trauma symptoms — before and after that experience.
Still, Williams says addressing racism is much more complex than treating racial trauma. "One of the questions I get asked a lot is, 'How can Black people cope with racism?' And I don't really like that question," she says. "I think it's important and I don't mind answering it, but I think the more important question is, how can we end racism? What can Black people do to stop racism that's happening to them and what can we do as a society to stop racism? And people aren't really asking this question."
The future of non-hormonal birth control: Antibodies can stop sperm in their tracks
Many women want non-hormonal birth control. A 22-year-old's findings were used to launch a company that could, within the decade, bring a new kind of contraceptive to the marketplace.
Unwanted pregnancy can now be added to the list of preventions that antibodies may be fighting in the near future. For decades, really since the 1980s, engineered monoclonal antibodies have been knocking out invading germs — preventing everything from cancer to COVID. Sperm, which have some of the same properties as germs, may be next.
Not only is there an unmet need on the market for alternatives to hormonal contraceptives, the genesis for the original research was personal for the then 22-year-old scientist who led it. Her findings were used to launch a company that could, within the decade, bring a new kind of contraceptive to the marketplace.
The genesis
It’s Suruchi Shrestha’s research — published in Science Translational Medicine in August 2021 and conducted as part of her dissertation while she was a graduate student at the University of North Carolina at Chapel Hill — that could change the future of contraception for many women worldwide. According to a Guttmacher Institute report, in the U.S. alone, there were 46 million sexually active women of reproductive age (15–49) who did not want to get pregnant in 2018. With the overturning of Roe v. Wade last year, Shrestha’s research could, indeed, be life changing for millions of American women and their families.
Now a scientist with NextVivo, Shrestha is not directly involved in the development of the contraceptive that is based on her research. But, back in 2016 when she was going through her own problems with hormonal contraceptives, she “was very personally invested” in her research project, Shrestha says. She was coping with a long list of negative effects from an implanted hormonal IUD. According to the Mayo Clinic, those can include severe pelvic pain, headaches, acute acne, breast tenderness, irregular bleeding and mood swings. After a year, she had the IUD removed, but it took another full year before all the side effects finally subsided; she also watched her sister suffer the “same tribulations” after trying a hormonal IUD, she says.
For contraceptive use either daily or monthly, Shrestha says, “You want the antibody to be very potent and also cheap.” That was her goal when she launched her study.
Shrestha unshelved antibody research that had been sitting idle for decades. It was in the late 80s that scientists in Japan first tried to develop anti-sperm antibodies for contraceptive use. But, 35 years ago, “Antibody production had not been streamlined as it is now, so antibodies were very expensive,” Shrestha explains. So, they shifted away from birth control, opting to focus on developing antibodies for vaccines.
Over the course of the last three decades, different teams of researchers have been working to make the antibody more effective, bringing the cost down, though it’s still expensive, according to Shrestha. For contraceptive use either daily or monthly, she says, “You want the antibody to be very potent and also cheap.” That was her goal when she launched her study.
The problem
The problem with contraceptives for women, Shrestha says, is that all but a few of them are hormone-based or have other negative side effects. In fact, some studies and reports show that millions of women risk unintended pregnancy because of medical contraindications with hormone-based contraceptives or to avoid the risks and side effects. While there are about a dozen contraceptive choices for women, there are two for men: the condom, considered 98% effective if used correctly, and vasectomy, 99% effective. Neither of these choices are hormone-based.
On the non-hormonal side for women, there is the diaphragm which is considered only 87 percent effective. It works better with the addition of spermicides — Nonoxynol-9, or N-9 — however, they are detergents; they not only kill the sperm, they also erode the vaginal epithelium. And, there’s the non-hormonal IUD which is 99% effective. However, the IUD needs to be inserted by a medical professional, and it has a number of negative side effects, including painful cramping at a higher frequency and extremely heavy or “abnormal” and unpredictable menstrual flows.
The hormonal version of the IUD, also considered 99% effective, is the one Shrestha used which caused her two years of pain. Of course, there’s the pill, which needs to be taken daily, and the birth control ring which is worn 24/7. Both cause side effects similar to the other hormonal contraceptives on the market. The ring is considered 93% effective mostly because of user error; the pill is considered 99% effective if taken correctly.
“That’s where we saw this opening or gap for women. We want a safe, non-hormonal contraceptive,” Shrestha says. Compounding the lack of good choices, is poor access to quality sex education and family planning information, according to the non-profit Urban Institute. A focus group survey suggested that the sex education women received “often lacked substance, leaving them feeling unprepared to make smart decisions about their sexual health and safety,” wrote the authors of the Urban Institute report. In fact, nearly half (45%, or 2.8 million) of the pregnancies that occur each year in the US are unintended, reports the Guttmacher Institute. Globally the numbers are similar. According to a new report by the United Nations, each year there are 121 million unintended pregnancies, worldwide.
The science
The early work on antibodies as a contraceptive had been inspired by women with infertility. It turns out that 9 to 12 percent of women who are treated for infertility have antibodies that develop naturally and work against sperm. Shrestha was encouraged that the antibodies were specific to the target — sperm — and therefore “very safe to use in women.” She aimed to make the antibodies more stable, more effective and less expensive so they could be more easily manufactured.
Since antibodies tend to stick to things that you tell them to stick to, the idea was, basically, to engineer antibodies to stick to sperm so they would stop swimming. Shrestha and her colleagues took the binding arm of an antibody that they’d isolated from an infertile woman. Then, targeting a unique surface antigen present on human sperm, they engineered a panel of antibodies with as many as six to 10 binding arms — “almost like tongs with prongs on the tongs, that bind the sperm,” explains Shrestha. “We decided to add those grabbers on top of it, behind it. So it went from having two prongs to almost 10. And the whole goal was to have so many arms binding the sperm that it clumps it” into a “dollop,” explains Shrestha, who earned a patent on her research.

Suruchi Shrestha works in the lab with a colleague. In 2016, her research on antibodies for birth control was inspired by her own experience with side effects from an implanted hormonal IUD.
UNC - Chapel Hill
The sperm stays right where it met the antibody, never reaching the egg for fertilization. Eventually, and naturally, “Our vaginal system will just flush it out,” Shrestha explains.
“She showed in her early studies that [she] definitely got the sperm immotile, so they didn't move. And that was a really promising start,” says Jasmine Edelstein, a scientist with an expertise in antibody engineering who was not involved in this research. Shrestha’s team at UNC reproduced the effect in the sheep, notes Edelstein, who works at the startup Be Biopharma. In fact, Shrestha’s anti-sperm antibodies that caused the sperm to agglutinate, or clump together, were 99.9% effective when delivered topically to the sheep’s reproductive tracts.
The future
Going forward, Shrestha thinks the ideal approach would be delivering the antibodies through a vaginal ring. “We want to use it at the source of the spark,” Shrestha says, as opposed to less direct methods, such as taking a pill. The ring would dissolve after one month, she explains, “and then you get another one.”
Engineered to have a long shelf life, the anti-sperm antibody ring could be purchased without a prescription, and women could insert it themselves, without a doctor. “That's our hope, so that it is accessible,” Shrestha says. “Anybody can just go and grab it and not worry about pregnancy or unintended pregnancy.”
Her patented research has been licensed by several biotech companies for clinical trials. A number of Shrestha’s co-authors, including her lab advisor, Sam Lai, have launched a company, Mucommune, to continue developing the contraceptives based on these antibodies.
And, results from a small clinical trial run by researchers at Boston University Chobanian & Avedisian School of Medicine show that a dissolvable vaginal film with antibodies was safe when tested on healthy women of reproductive age. That same group of researchers last year received a $7.2 million grant from the National Institute of Health for further research on monoclonal antibody-based contraceptives, which have also been shown to block transmission of viruses, like HIV.
“As the costs come down, this becomes a more realistic option potentially for women,” says Edelstein. “The impact could be tremendous.”
This article was first published by Leaps.org in December, 2022. It has been lightly edited with updates for timeliness.
Researchers probe extreme gene therapy for severe alcoholism
When all traditional therapeutic approaches fail for alcohol abuse disorder, a radical gene therapy might be something to try in the future.
Story by Freethink
A single shot — a gene therapy injected into the brain — dramatically reduced alcohol consumption in monkeys that previously drank heavily. If the therapy is safe and effective in people, it might one day be a permanent treatment for alcoholism for people with no other options.
The challenge: Alcohol use disorder (AUD) means a person has trouble controlling their alcohol consumption, even when it is negatively affecting their life, job, or health.
In the U.S., more than 10 percent of people over the age of 12 are estimated to have AUD, and while medications, counseling, or sheer willpower can help some stop drinking, staying sober can be a huge struggle — an estimated 40-60 percent of people relapse at least once.
A team of U.S. researchers suspected that an in-development gene therapy for Parkinson’s disease might work as a dopamine-replenishing treatment for alcoholism, too.
According to the CDC, more than 140,000 Americans are dying each year from alcohol-related causes, and the rate of deaths has been rising for years, especially during the pandemic.
The idea: For occasional drinkers, alcohol causes the brain to release more dopamine, a chemical that makes you feel good. Chronic alcohol use, however, causes the brain to produce, and process, less dopamine, and this persistent dopamine deficit has been linked to alcohol relapse.
There is currently no way to reverse the changes in the brain brought about by AUD, but a team of U.S. researchers suspected that an in-development gene therapy for Parkinson’s disease might work as a dopamine-replenishing treatment for alcoholism, too.
To find out, they tested it in heavy-drinking monkeys — and the animals’ alcohol consumption dropped by 90% over the course of a year.
How it works: The treatment centers on the protein GDNF (“glial cell line-derived neurotrophic factor”), which supports the survival of certain neurons, including ones linked to dopamine.
For the new study, a harmless virus was used to deliver the gene that codes for GDNF into the brains of four monkeys that, when they had the option, drank heavily — the amount of ethanol-infused water they consumed would be equivalent to a person having nine drinks per day.
“We targeted the cell bodies that produce dopamine with this gene to increase dopamine synthesis, thereby replenishing or restoring what chronic drinking has taken away,” said co-lead researcher Kathleen Grant.
To serve as controls, another four heavy-drinking monkeys underwent the same procedure, but with a saline solution delivered instead of the gene therapy.
The results: All of the monkeys had their access to alcohol removed for two months following the surgery. When it was then reintroduced for four weeks, the heavy drinkers consumed 50 percent less compared to the control group.
When the researchers examined the monkeys’ brains at the end of the study, they were able to confirm that dopamine levels had been replenished in the treated animals, but remained low in the controls.
The researchers then took the alcohol away for another four weeks, before giving it back for four. They repeated this cycle for a year, and by the end of it, the treated monkeys’ consumption had fallen by more than 90 percent compared to the controls.
“Drinking went down to almost zero,” said Grant. “For months on end, these animals would choose to drink water and just avoid drinking alcohol altogether. They decreased their drinking to the point that it was so low we didn’t record a blood-alcohol level.”
When the researchers examined the monkeys’ brains at the end of the study, they were able to confirm that dopamine levels had been replenished in the treated animals, but remained low in the controls.
Looking ahead: Dopamine is involved in a lot more than addiction, so more research is needed to not only see if the results translate to people but whether the gene therapy leads to any unwanted changes to mood or behavior.
Because the therapy requires invasive brain surgery and is likely irreversible, it’s unlikely to ever become a common treatment for alcoholism — but it could one day be the only thing standing between people with severe AUD and death.
“[The treatment] would be most appropriate for people who have already shown that all our normal therapeutic approaches do not work for them,” said Grant. “They are likely to create severe harm or kill themselves or others due to their drinking.”
This article originally appeared on Freethink, home of the brightest minds and biggest ideas of all time.


