Rehabilitating psychedelic drugs: Another key to treating severe mental health disorders

A recent review paper found evidence that using psychedelics such as MDMA can help with treating a variety of common mental illnesses, but experts fear that research might easily be shut down in the future.
Lori Tipton's life was a cascade of trauma that even a soap opera would not dare inflict upon a character: a mentally unstable family; a brother who died of a drug overdose; the shocking discovery of the bodies of two persons her mother had killed before turning the gun on herself; the devastation of Hurricane Katrina that savaged her hometown of New Orleans; being raped by someone she trusted; and having an abortion. She suffered from severe PTSD.
“My life was filled with anxiety and hypervigilance,” she says. “I was constantly afraid and had mood swings, panic attacks, insomnia, intrusive thoughts and suicidal ideation. I tried to take my life more than once.” She was fortunate to be able to access multiple mental health services, “And while at times some of these modalities would relieve the symptoms, nothing really lasted and nothing really address the core trauma.”
Then in 2018 Tipton enrolled in a clinical trial that combined intense sessions of psychotherapy with limited use of Methylenedioxymethamphetamine, or MDMA, a drug classified as a psychedelic and commonly known as ecstasy or Molly. The regimen was arduous; 1-2 hour preparation sessions, three sessions where MDMA was used, which lasted 6-8 hours, and lengthy sessions afterward to process and integrate the experiences. Two therapists were with her every moment of the three-month program that totaled more than 40 hours.
“It was clear to me that [the therapists] weren't going to heal me, that I was going to have to do the work for myself, but that they were there to completely support my process,” she says. “But the effects of MDMA were really undeniable for me. I felt embodied in a way that I hadn't in years. PTSD had robbed me of the ability to feel safe in my own body.”
Tipton doesn’t think the therapy completely cured her PTSD. “But when I completed the trial in 2018, I no longer qualified for the diagnosis, and I still don't qualify for the diagnosis today,” she told an April workshop on psychedelics as mental health treatment by the National Academies of Sciences, Engineering and Medicine, or NASEM.
A Champion
Rick Doblin has been a catalyst behind much of the contemporary research into psychedelics. Prior to the DEA clamp down, the Boston psychotherapist had seen that MDMA and other psychedelics could benefit some of his patients where other measures had failed. He immediately organized efforts to question the drug rescheduling but to little avail. In 1986, he created the nonprofit Multidisciplinary Association for Psychedelic Studies (MAPS), which slowly laid the scientific foundation for clinical trials, including the one that Tipton joined, using psychedelics to treat mental health conditions.
Now, only slowly, have researchers been able to explore the power of these drugs to treat a broad spectrum of severely debilitating mental health conditions, including trauma, depression, and PTSD, where other available treatments proved inadequate.
“Psychedelic psychotherapy is an attempt to go after the root causes of the problems with just a relatively few administrations, as contrasted to most of the psychiatric drugs used today that are mostly just reducing symptoms and are meant to be taken on a daily basis,” Doblin said in a 2019 TED Talk. Most of these drugs can have broad effect but “some are probably more effective than others for certain conditions,” he added in a recent interview with Leaps.org. Comparative head-to-head studies of psychedelic therapies simply have not been conducted.
Their mechanisms of action are poorly understood and can vary between drugs, but it is generally believed that psychedelics change the activity of neurons so that the brain processes information differently, says Katrin Preller, a neuropsychologist at the University of Zurich. A recent important study in Nature Medicine by Richard Daws and colleagues used functional magnetic resonance imaging (fMRI) of the brain and found that “functional networks became more functionally interconnected and flexible after psilocybin treatment…implying that psilocybin's antidepressant action may depend on a global increase in brain network integration.”
Rosalind Watts, a clinical investigator at the Imperial College in London, believes there is “an overestimation of the importance of the drug and an underestimation of the importance of the [therapeutic] context” in psychedelic research. “It is unethical to provide the drug without the other,” she says. Doblin notes that “psychotherapy outcomes research demonstrates that the therapeutic alliance between the therapist and the patients is the single most predictive factor of outcomes. [It is] trust and the sense of safety, the willingness to go into difficult spaces” that makes clinical breakthroughs possible with the drug.
Excitement and Challenges
Recurrent themes expressed at the NASEM workshop were exciting glimpses of the potential for psychedelics to treat mental health conditions combined with the challenges of realizing those potentials. A recent review paper found evidence that using psychedelics can help with treating a variety of common mental illnesses, but the paper could identify only 14 clinical trials of classic psychedelics published since 1991. Much of the reason is that the drugs are not patentable and so the pharmaceutical industry has no interest in investing in expensive clinical trials to bring them to market. MAPS has raised about $135 million over its 36-year history to conduct such research, says Doblin, the vast majority of it from individual donors and none from foundations.
The workshop participants’ views also were colored by the history of drug crackdowns and a fear that research might easily be shut down in the future. There was great concern that use of psychedelics should be confined to clinical trials with high safety and ethical standards, instead of doctors and patients experimenting on their own. “We need to get it right this time,” says Charles Grob, a psychiatrist at the UCLA School of Medicine. But restricting access to psychedelics will become even more difficult now that Oregon and several cities have acted to decriminalize possession and use of many of these drugs.
The experience with ketamine also troubled Grob. He is hoping to “mitigate the rush of rapid commercialization” that occurred with that drug. Ketamine technically is not a psychedelic though it does share some of their potentially euphoric properties. In 2019, soon after the FDA approved a form of ketamine with a limited label indication to treat depression, for profit clinics sprang up promoting off label use of the drug for psychiatric conditions where there was little clinical evidence of efficacy. He fears the same thing will happen when true psychedelics are made available.
If these therapies are approved, access to them is likely to be a problem. The drugs themselves are cheap but the accompanying therapy is not, and there is a shortage of trained psychotherapists. Mental health services often are not adequately covered by health insurance, while the poor and people of color suffer additional burdens of inadequate access. Doblin is committed to health care equity by training additional providers and by investigating whether some of the preparatory and integration sessions might be handled in a group setting. He says it is important that the legal aspects of psychedelics also be addressed so that patients “don't have to go underground” in order to receive this care.
Why you should (virtually) care
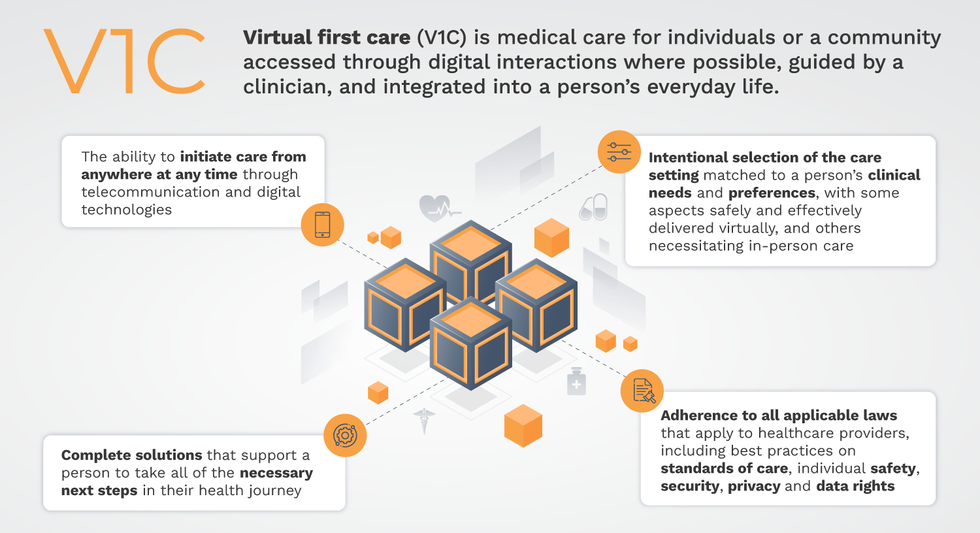
Virtual-first care, or V1C, could increase the quality of healthcare and make it more patient-centric by letting patients combine in-person visits with virtual options such as video for seeing their care providers.
As the pandemic turns endemic, healthcare providers have been eagerly urging patients to return to their offices to enjoy the benefits of in-person care.
But wait.
The last two years have forced all sorts of organizations to be nimble, adaptable and creative in how they work, and this includes healthcare providers’ efforts to maintain continuity of care under the most challenging of conditions. So before we go back to “business as usual,” don’t we owe it to those providers and ourselves to admit that business as usual did not work for most of the people the industry exists to help? If we’re going to embrace yet another period of change – periods that don’t happen often in our complex industry – shouldn’t we first stop and ask ourselves what we’re trying to achieve?
Certainly, COVID has shown that telehealth can be an invaluable tool, particularly for patients in rural and underserved communities that lack access to specialty care. It’s also become clear that many – though not all – healthcare encounters can be effectively conducted from afar. That said, the telehealth tactics that filled the gap during the pandemic were largely stitched together substitutes for existing visit-based workflows: with offices closed, patients scheduled video visits for help managing the side effects of their blood pressure medications or to see their endocrinologist for a quarterly check-in. Anyone whose children slogged through the last year or two of remote learning can tell you that simply virtualizing existing processes doesn’t necessarily improve the experience or the outcomes!
But what if our approach to post-pandemic healthcare came from a patient-driven perspective? We have a fleeting opportunity to advance a care model centered on convenient and equitable access that first prioritizes good outcomes, then selects approaches to care – and locations – tailored to each patient. Using the example of education, imagine how effective it would be if each student, regardless of their school district and aptitude, received such individualized attention.
That’s the idea behind virtual-first care (V1C), a new care model centered on convenient, customized, high-quality care that integrates a full suite of services tailored directly to patients’ clinical needs and preferences. This package includes asynchronous communication such as texting; video and other live virtual modes; and in-person options.
V1C goes beyond what you might think of as standard “telehealth” by using evidence-based protocols and tools that include traditional and digital therapeutics and testing, personalized care plans, dynamic patient monitoring, and team-based approaches to care. This could include spit kits mailed for laboratory tests and complementing clinical care with health coaching. V1C also replaces some in-person exams with ongoing monitoring, using sensors for more ‘whole person’ care.
Amidst all this momentum, we have the opportunity to rethink the goals of healthcare innovation, but that means bringing together key stakeholders to demonstrate that sustainable V1C can redefine healthcare.
Established V1C healthcare providers such as Omada, Headspace, and Heartbeat Health, as well as emerging market entrants like Oshi, Visana, and Wellinks, work with a variety of patients who have complicated long-term conditions such as diabetes, heart failure, gastrointestinal illness, endometriosis, and COPD. V1C is comprehensive in ways that are lacking in digital health and its other predecessors: it has the potential to integrate multiple data streams, incorporate more frequent touches and check-ins over time, and manage a much wider range of chronic health conditions, improving lives and reducing disease burden now and in the future.
Recognizing the pandemic-driven interest in virtual care, significant energy and resources are already flowing fast toward V1C. Some of the world’s largest innovators jumped into V1C early on: Verily, Alphabet’s Life Sciences Company, launched Onduo in 2016 to disrupt the diabetes healthcare market, and is now well positioned to scale its solutions. Major insurers like Aetna and United now offer virtual-first plans to members, responding as organizations expand virtual options for employees. Amidst all this momentum, we have the opportunity to rethink the goals of healthcare innovation, but that means bringing together key stakeholders to demonstrate that sustainable V1C can redefine healthcare.
That was the immediate impetus for IMPACT, a consortium of V1C companies, investors, payers and patients founded last year to ensure access to high-quality, evidence-based V1C. Developed by our team at the Digital Medicine Society (DiMe) in collaboration with the American Telemedicine Association (ATA), IMPACT has begun to explore key issues that include giving patients more integrated experiences when accessing both virtual and brick-and-mortar care.
Digital Medicine Society
V1C is not, nor should it be, virtual-only care. In this new era of hybrid healthcare, success will be defined by how well providers help patients navigate the transitions. How do we smoothly hand a patient off from an onsite primary care physician to, say, a virtual cardiologist? How do we get information from a brick-and-mortar to a digital portal? How do you manage dataflow while still staying HIPAA compliant? There are many complex regulatory implications for these new models, as well as an evolving landscape in terms of privacy, security and interoperability. It will be no small task for groups like IMPACT to determine the best path forward.
None of these factors matter unless the industry can recruit and retain clinicians. Our field is facing an unprecedented workforce crisis. Traditional healthcare is making clinicians miserable, and COVID has only accelerated the trend of overworked, disenchanted healthcare workers leaving in droves. Clinicians want more interactions with patients, and fewer with computer screens – call it “More face time, less FaceTime.” No new model will succeed unless the industry can more efficiently deploy its talent – arguably its most scarce and precious resource. V1C can help with alleviating the increasing burden and frustration borne by individual physicians in today’s status quo.
In healthcare, new technological approaches inevitably provoke no shortage of skepticism. Past lessons from Silicon Valley-driven fixes have led to understandable cynicism. But V1C is a different breed of animal. By building healthcare around the patient, not the clinic, V1C can make healthcare work better for patients, payers and providers. We’re at a fork in the road: we can revert back to a broken sick-care system, or dig in and do the hard work of figuring out how this future-forward healthcare system gets financed, organized and executed. As a field, we must find the courage and summon the energy to embrace this moment, and make it a moment of change.
Podcast: The future of brain health with Percy Griffin
Percy Griffin, director of scientific engagement for the Alzheimer’s Association, joins Leaps.org to discuss the present and future of the fight against dementia.
Today's guest is Percy Griffin, director of scientific engagement for the Alzheimer’s Association, a nonprofit that’s focused on speeding up research, finding better ways to detect Alzheimer’s earlier and other approaches for reducing risk. Percy has a doctorate in molecular cell biology from Washington University, he’s led important research on Alzheimer’s, and you can find the link to his full bio in the show notes, below.
Our topic for this conversation is the present and future of the fight against dementia. Billions of dollars have been spent by the National Institutes of Health and biotechs to research new treatments for Alzheimer's and other forms of dementia, but so far there's been little to show for it. Last year, Aduhelm became the first drug to be approved by the FDA for Alzheimer’s in 20 years, but it's received a raft of bad publicity, with red flags about its effectiveness, side effects and cost.
Meanwhile, 6.5 million Americans have Alzheimer's, and this number could increase to 13 million in 2050. Listen to this conversation if you’re concerned about your own brain health, that of family members getting older, or if you’re just concerned about the future of this country with experts predicting the number people over 65 will increase dramatically in the very near future.
Listen to the Episode
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
4:40 - We talk about the parts of Percy’s life that led to him to concentrate on working in this important area.
6:20 - He defines Alzheimer's and dementia, and discusses the key elements of communicating science.
10:20 - Percy explains why the Alzheimer’s Association has been supportive of Aduhelm, even as others have been critical.
17:58 - We talk about therapeutics under development, which ones to be excited about, and how they could be tailored to a person's own biology.
24:25 - Percy discusses funding and tradeoffs between investing more money into Alzheimer’s research compared to other intractable diseases like cancer, and new opportunities to accelerate progress, such as ARPA-H, President Biden’s proposed agency to speed up health breakthroughs.
27:24 - We talk about the social determinants of brain health. What are the pros/cons of continuing to spend massive sums of money to develop new drugs like Aduhelm versus refocusing on expanding policies to address social determinants - like better education, nutritious food and safe drinking water - that have enabled some groups more than others to enjoy improved cognition late in life.
34:18 - Percy describes his top lifestyle recommendations for protecting your mind.
37:33 - Is napping bad for the brain?
39:39 - Circadian rhythm and Alzheimer's.
42:34 - What tests can people take to check their brain health today, and which biomarkers are we making progress on?
47:25 - Percy highlights important programs run by the Alzheimer’s Association to support advances.
Show links:
** After this episode was recorded, the Centers for Medicare and Medicaid Services affirmed its decision from last June to limit coverage of Aduhelm. More here.
- Percy Griffin's bio: https://www.alz.org/manh/events/alztalks/upcoming-...
- The Alzheimer's Association's Part the Cloud program: https://alz.org/partthecloud/about-us.asp
- The paradox of dementia rates decreasing: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC7455342/
- The argument for focusing more resources on improving institutions and social processes for brain health: https://www.statnews.com/2021/09/23/the-brain-heal...
- Recent research on napping: https://www.ocregister.com/2022/03/25/alzheimers-s...
- The Alzheimer's Association helpline: https://www.alz.org/help-support/resources/helpline
- ALZConnected, a free online community for people affected by dementia https://www.alzconnected.org/
- TrialMatch for people with dementia and healthy volunteers to find clinical trials for Alzheimer's and other dementia: https://www.alz.org/alzheimers-dementia/research_p...

