Can AI help create “smart borders” between countries?
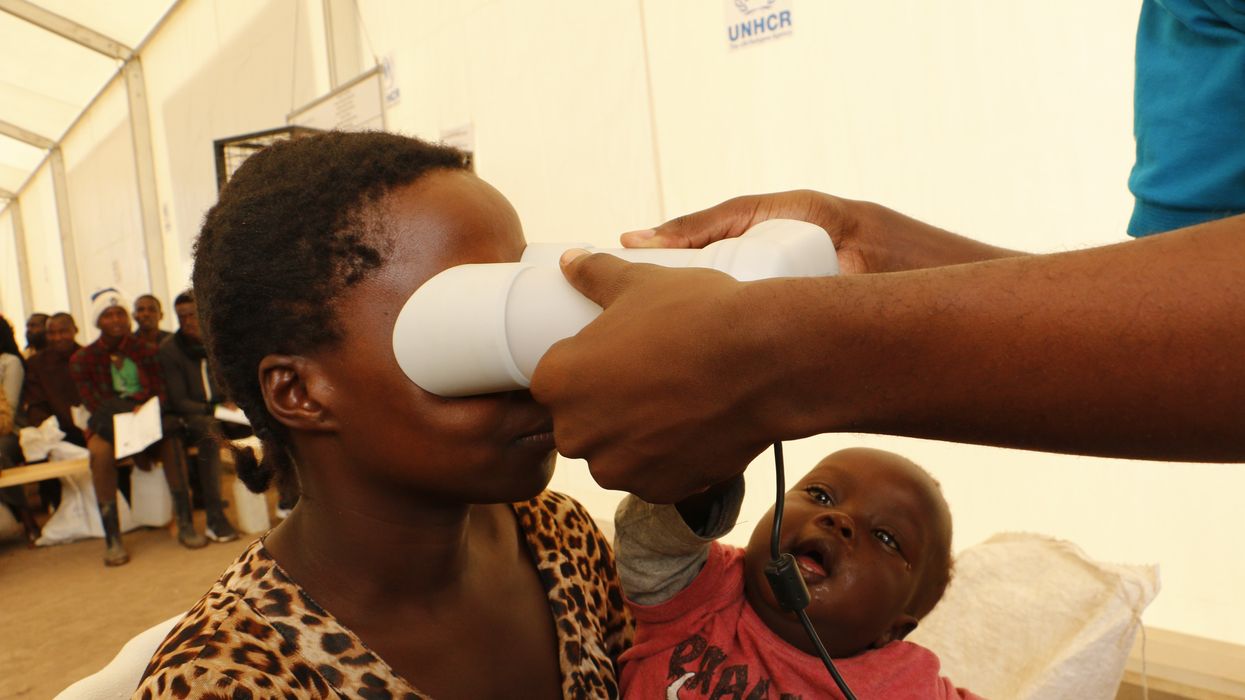
A refugee in Uganda’s Oruchinga settlement uses an iris scan to claim food assistance.
In 2016, border patrols in Greece, Latvia and Hungary received a prototype for an AI-powered lie detector to help screen asylum seekers. The detector, called iBorderCtrl, was funded by the European Commission in hopes to eventually mitigate refugee crises like the one sparked by the Syrian civil war a year prior.
iBorderCtrl, which analyzes micro expressions in the face, received but one slice of the Commission’s €34.9 billion border control and migration management budget. Still in development is the more ambitious EuMigraTool, a predictive AI system that will process internet news and social media posts to estimate not only the number of migrants heading for a particular country, but also the “risks of tensions between migrants and EU citizens.”
Both iBorderCtrl and EuMigraTool are part of a broader trend: the growing digitization of migration-related technologies. Outside of the EU, in refugee camps in Jordan, the United Nations introduced iris scanning software to distribute humanitarian aid, including food and medicine. And in the United States, Customs and Border Protection has attempted to automate its services through an app called CBP One, which both travelers and asylum seekers can use to apply for I-94 forms, the arrival-departure record cards for people who are not U.S. citizens or permanent residents.
According to Koen Leurs, professor of gender, media and migration studies at Utrecht University in the Netherlands, we have arrived at a point where migration management has become so reliant on digital technology that the former can no longer be studied in isolation from the latter. Investigating this reliance for his new book, Digital Migration, Leurs came to the conclusion that applications like those mentioned above are more often than not a double-edged sword, presenting both benefits and drawbacks.
There has been “a huge acceleration” in the way digital technologies “dehumanize people,” says Koen Leurs, professor of gender, media and migration studies at Utrecht University in the Netherlands. Governments treat asylum seekers as test subjects for new inventions, all along the borders of the developed world.
On the one hand, digital technology can make migration management more efficient and less labor intensive, enabling countries to process larger numbers of people in a time when global movement is on the rise due to globalization and political instability. Leurs also discovered that informal knowledge networks such as Informed Immigrant, an online resource that connects migrants to social workers and community organizers, have positively impacted the lives of their users. The same, Leurs notes, is true of platforms like Twitter, Facebook, and WhatsApp, all of which migrants use to stay in touch with each other as well as their families back home. “The emotional support you receive through social media is something we all came to appreciate during the COVID pandemic,” Leurs says. “For refugees, this had already been common knowledge for years.”
On the flipside, automatization of migration management – particularly through the use of AI – has spawned extensive criticism from human rights activists. Sharing their sentiment, Leurs attests that many so-called innovations are making life harder for migrants, not easier. He also says there has been “a huge acceleration” in the way digital technologies “dehumanize people,” and that governments treat asylum seekers as test subjects for new inventions, all along the borders of the developed world.
In Jordan, for example, refugees had to scan their irises in order to collect aid, prompting the question of whether such measures are ethical. Speaking to Reuters, Petra Molnar, a fellow at Harvard University’s Berkman Klein Center for Internet and Society, said that she was troubled by the fact that this experiment was done on marginalized people. “The refugees are guinea pigs,” she said. “Imagine what would happen at your local grocery store if all of a sudden iris scanning became a thing,” she pointed out. “People would be up in arms. But somehow it is OK to do it in a refugee camp.”
Artificial intelligence programs have been scrutinized for their unreliability, their complex processing, thwarted by the race and gender biases picked up from training data. In 2019, a female reporter from The Intercept tested iBorderCtrl and, despite answering all questions truthfully, was accused by the machine of lying four out of 16 times. Had she been waiting at checkpoint on the Greek or Latvian border, she would have been flagged for additional screening – a measure that could jeopardize her chance of entry. Because of its biases, and the negative press that this attracted, iBorderCtrl did not move past its test phase.
While facial recognition caused problems on the European border, it was helpful in Ukraine, where programs like those developed by software company Clearview AI are used to spot Russian spies, identify dead soldiers, and check movement in and out of war zones.
In April 2021, not long after iBorderCtrl was shut down, the European Commission proposed the world’s first-ever legal framework for AI regulation: the Artificial Intelligence Act. The act, which is still being developed, promises to prevent potentially “harmful” AI practices from being used in migration management. In the most recent draft, approved by the European Parliament’s Liberties and Internal Market committees, the ban included emotion recognition systems (like iBorderCtrl), predictive policing systems (like EUMigraTool), and biometric categorization systems (like iris scanners). The act also stipulates that AI must be subject to strict oversight and accountability measures.
While some worry the AI Act is not comprehensive enough, others wonder if it is in fact going too far. Indeed, many proponents of machine learning argue that, by placing a categorical ban on certain systems, governments will thwart the development of potentially useful technology. While facial recognition caused problems on the European border, it was helpful in Ukraine, where programs like those developed by software company Clearview AI are used to spot Russian spies, identify dead soldiers, and check movement in and out of war zones.
Instead of flat-out banning AI, why not strive to make it more reliable? “One of the most compelling arguments against AI is that it is inherently biased,” says Vera Raposo, an assistant professor of law at NOVA University in Lisbon specializing in digital law. “In truth, AI itself is not biased; it becomes biased due to human influence. It seems that complete eradication of biases is unattainable, but mitigation is possible. We can strive to reduce biases by employing more comprehensive and unbiased data in AI training and encompassing a wider range of individuals. We can also work on developing less biased algorithms, although this is challenging given that coders, being human, inherently possess biases of their own.”
AI is most effective when it enhances human performance rather than replacing it.
Accessibility is another obstacle that needs to be overcome. Leurs points out that, in migration management, AI often functions as a “black box” because the migration officers operating it are unable to comprehend its complex decision-making process and thus unable to scrutinize its results. One solution to this problem is to have law enforcement work closely with AI experts. Alternatively, machine learning could be limited to gathering and summarizing information, leaving evaluation of that information to actual people.
Raposo agrees AI is most effective when it enhances human performance rather than replacing it. On the topic of transparency, she does note that making an AI that is both sophisticated and easy to understand is a little bit like having your cake and eating it too. “In numerous domains,” she explains, “we might need to accept a reduced level of explainability in exchange for a high degree of accuracy (assuming we cannot have both).” Using healthcare as an analogy, she adds that “some medications work in ways not fully understood by either doctors or pharma companies, yet persist due to demonstrated efficacy in clinical trials.”
Leurs believes digital technologies used in migration management can be improved through a push for more conscientious research. “Technology is a poison and a medicine for that poison,” he argues, which is why new tech should be developed with its potential applications in mind. “Ethics has become a major concern in recent years. Increasingly, and particularly in the study of forced migration, researchers are posing critical questions like ‘what happens with the data that is gathered?’ and ‘who will this harm?’” In some cases, Leurs thinks, that last question may need to be reversed: we should be thinking about how we can actively disarm oppressive structures. “After all, our work should align with the interests of the communities it is going to affect.”
Jamie Rettinger with his now fiance Amie Purnel-Davis, who helped him through the clinical trial.
Jamie Rettinger was still in his thirties when he first noticed a tiny streak of brown running through the thumbnail of his right hand. It slowly grew wider and the skin underneath began to deteriorate before he went to a local dermatologist in 2013. The doctor thought it was a wart and tried scooping it out, treating the affected area for three years before finally removing the nail bed and sending it off to a pathology lab for analysis.
"I have some bad news for you; what we removed was a five-millimeter melanoma, a cancerous tumor that often spreads," Jamie recalls being told on his return visit. "I'd never heard of cancer coming through a thumbnail," he says. None of his doctors had ever mentioned it either. "I just thought I was being treated for a wart." But nothing was healing and it continued to bleed.
A few months later a surgeon amputated the top half of his thumb. Lymph node biopsy tested negative for spread of the cancer and when the bandages finally came off, Jamie thought his medical issues were resolved.
Melanoma is the deadliest form of skin cancer. About 85,000 people are diagnosed with it each year in the U.S. and more than 8,000 die of the cancer when it spreads to other parts of the body, according to the Centers for Disease Control and Prevention (CDC).
There are two peaks in diagnosis of melanoma; one is in younger women ages 30-40 and often is tied to past use of tanning beds; the second is older men 60+ and is related to outdoor activity from farming to sports. Light-skinned people have a twenty-times greater risk of melanoma than do people with dark skin.
"When I graduated from medical school, in 2005, melanoma was a death sentence" --Diwakar Davar.
Jamie had a follow up PET scan about six months after his surgery. A suspicious spot on his lung led to a biopsy that came back positive for melanoma. The cancer had spread. Treatment with a monoclonal antibody (nivolumab/Opdivo®) didn't prove effective and he was referred to the UPMC Hillman Cancer Center in Pittsburgh, a four-hour drive from his home in western Ohio.
An alternative monoclonal antibody treatment brought on such bad side effects, diarrhea as often as 15 times a day, that it took more than a week of hospitalization to stabilize his condition. The only options left were experimental approaches in clinical trials.
Early research
"When I graduated from medical school, in 2005, melanoma was a death sentence" with a cure rate in the single digits, says Diwakar Davar, 39, an oncologist at UPMC Hillman Cancer Center who specializes in skin cancer. That began to change in 2010 with introduction of the first immunotherapies, monoclonal antibodies, to treat cancer. The antibodies attach to PD-1, a receptor on the surface of T cells of the immune system and on cancer cells. Antibody treatment boosted the melanoma cure rate to about 30 percent. The search was on to understand why some people responded to these drugs and others did not.
At the same time, there was a growing understanding of the role that bacteria in the gut, the gut microbiome, plays in helping to train and maintain the function of the body's various immune cells. Perhaps the bacteria also plays a role in shaping the immune response to cancer therapy.
One clue came from genetically identical mice. Animals ordered from different suppliers sometimes responded differently to the experiments being performed. That difference was traced to different compositions of their gut microbiome; transferring the microbiome from one animal to another in a process known as fecal transplant (FMT) could change their responses to disease or treatment.
When researchers looked at humans, they found that the patients who responded well to immunotherapies had a gut microbiome that looked like healthy normal folks, but patients who didn't respond had missing or reduced strains of bacteria.
Davar and his team knew that FMT had a very successful cure rate in treating the gut dysbiosis of Clostridioides difficile, a persistant intestinal infection, and they wondered if a fecal transplant from a patient who had responded well to cancer immunotherapy treatment might improve the cure rate of patients who did not originally respond to immunotherapies for melanoma.
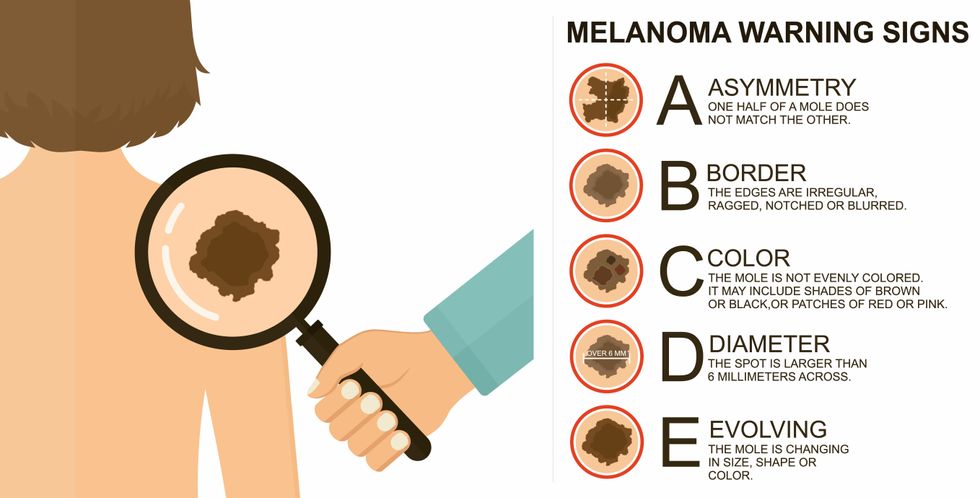
The ABCDE of melanoma detection
Adobe Stock
Clinical trial
"It was pretty weird, I was totally blasted away. Who had thought of this?" Jamie first thought when the hypothesis was explained to him. But Davar's explanation that the procedure might restore some of the beneficial bacterial his gut was lacking, convinced him to try. He quickly signed on in October 2018 to be the first person in the clinical trial.
Fecal donations go through the same safety procedures of screening for and inactivating diseases that are used in processing blood donations to make them safe for transfusion. The procedure itself uses a standard hollow colonoscope designed to screen for colon cancer and remove polyps. The transplant is inserted through the center of the flexible tube.
Most patients are sedated for procedures that use a colonoscope but Jamie doesn't respond to those drugs: "You can't knock me out. I was watching them on the TV going up my own butt. It was kind of unreal at that point," he says. "There were about twelve people in there watching because no one had seen this done before."
A test two weeks after the procedure showed that the FMT had engrafted and the once-missing bacteria were thriving in his gut. More importantly, his body was responding to another monoclonal antibody (pembrolizumab/Keytruda®) and signs of melanoma began to shrink. Every three months he made the four-hour drive from home to Pittsburgh for six rounds of treatment with the antibody drug.
"We were very, very lucky that the first patient had a great response," says Davar. "It allowed us to believe that even though we failed with the next six, we were on the right track. We just needed to tweak the [fecal] cocktail a little better" and enroll patients in the study who had less aggressive tumor growth and were likely to live long enough to complete the extensive rounds of therapy. Six of 15 patients responded positively in the pilot clinical trial that was published in the journal Science.
Davar believes they are beginning to understand the biological mechanisms of why some patients initially do not respond to immunotherapy but later can with a FMT. It is tied to the background level of inflammation produced by the interaction between the microbiome and the immune system. That paper is not yet published.
Surviving cancer
It has been almost a year since the last in his series of cancer treatments and Jamie has no measurable disease. He is cautiously optimistic that his cancer is not simply in remission but is gone for good. "I'm still scared every time I get my scans, because you don't know whether it is going to come back or not. And to realize that it is something that is totally out of my control."
"It was hard for me to regain trust" after being misdiagnosed and mistreated by several doctors he says. But his experience at Hillman helped to restore that trust "because they were interested in me, not just fixing the problem."
He is grateful for the support provided by family and friends over the last eight years. After a pause and a sigh, the ruggedly built 47-year-old says, "If everyone else was dead in my family, I probably wouldn't have been able to do it."
"I never hesitated to ask a question and I never hesitated to get a second opinion." But Jamie acknowledges the experience has made him more aware of the need for regular preventive medical care and a primary care physician. That person might have caught his melanoma at an earlier stage when it was easier to treat.
Davar continues to work on clinical studies to optimize this treatment approach. Perhaps down the road, screening the microbiome will be standard for melanoma and other cancers prior to using immunotherapies, and the FMT will be as simple as swallowing a handful of freeze-dried capsules off the shelf rather than through a colonoscopy. Earlier this year, the Food and Drug Administration approved the first oral fecal microbiota product for C. difficile, hopefully paving the way for more.
An older version of this hit article was first published on May 18, 2021
All organisms can repair damaged tissue, but none do it better than salamanders and newts. A surprising area of science could tell us how they manage this feat - and perhaps even help us develop a similar ability.
All organisms have the capacity to repair or regenerate tissue damage. None can do it better than salamanders or newts, which can regenerate an entire severed limb.
That feat has amazed and delighted man from the dawn of time and led to endless attempts to understand how it happens – and whether we can control it for our own purposes. An exciting new clue toward that understanding has come from a surprising source: research on the decline of cells, called cellular senescence.
Senescence is the last stage in the life of a cell. Whereas some cells simply break up or wither and die off, others transition into a zombie-like state where they can no longer divide. In this liminal phase, the cell still pumps out many different molecules that can affect its neighbors and cause low grade inflammation. Senescence is associated with many of the declining biological functions that characterize aging, such as inflammation and genomic instability.
Oddly enough, newts are one of the few species that do not accumulate senescent cells as they age, according to research over several years by Maximina Yun. A research group leader at the Center for Regenerative Therapies Dresden and the Max Planck Institute of Molecular and Cell Biology and Genetics, in Dresden, Germany, Yun discovered that senescent cells were induced at some stages of regeneration of the salamander limb, “and then, as the regeneration progresses, they disappeared, they were eliminated by the immune system,” she says. “They were present at particular times and then they disappeared.”
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states.
Previous research on senescence in aging had suggested, logically enough, that applying those cells to the stump of a newly severed salamander limb would slow or even stop its regeneration. But Yun stood that idea on its head. She theorized that senescent cells might also play a role in newt limb regeneration, and she tested it by both adding and removing senescent cells from her animals. It turned out she was right, as the newt limbs grew back faster than normal when more senescent cells were included.
Senescent cells added to the edges of the wound helped the healthy muscle cells to “dedifferentiate,” essentially turning back the developmental clock of those cells into more primitive states, which could then be turned into progenitors, a cell type in between stem cells and specialized cells, needed to regrow the muscle tissue of the missing limb. “We think that this ability to dedifferentiate is intrinsically a big part of why salamanders can regenerate all these very complex structures, which other organisms cannot,” she explains.
Yun sees regeneration as a two part problem. First, the cells must be able to sense that their neighbors from the lost limb are not there anymore. Second, they need to be able to produce the intermediary progenitors for regeneration, , to form what is missing. “Molecularly, that must be encoded like a 3D map,” she says, otherwise the new tissue might grow back as a blob, or liver, or fin instead of a limb.
Wound healing
Another recent study, this time at the Mayo Clinic, provides evidence supporting the role of senescent cells in regeneration. Looking closely at molecules that send information between cells in the wound of a mouse, the researchers found that senescent cells appeared near the start of the healing process and then disappeared as healing progressed. In contrast, persistent senescent cells were the hallmark of a chronic wound that did not heal properly. The function and significance of senescence cells depended on both the timing and the context of their environment.
The paper suggests that senescent cells are not all the same. That has become clearer as researchers have been able to identify protein markers on the surface of some senescent cells. The patterns of these proteins differ for some senescent cells compared to others. In biology, such physical differences suggest functional differences, so it is becoming increasingly likely there are subsets of senescent cells with differing functions that have not yet been identified.
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes.
Scientists initially thought that senescent cells couldn’t play a role in regeneration because they could no longer reproduce, says Anthony Atala, a practicing surgeon and bioengineer who leads the Wake Forest Institute for Regenerative Medicine in North Carolina. But Yun’s study points in the other direction. “What this paper shows clearly is that these cells have the potential to be involved in tissue regeneration [in newts]. The question becomes, will these cells be able to do the same in humans.”
As our knowledge of senescent cells increases, Atala thinks we need to embrace a new analogy to help understand them: humans in retirement. They “have acquired a lot of wisdom throughout their whole life and they can help younger people and mentor them to grow to their full potential. We're seeing the same thing with these cells,” he says. They are no longer putting energy into their own reproduction, but the signaling molecules they secrete “can help other cells around them to regenerate.”
There are disagreements within the research community as to whether newts have acquired their regenerative capacity through a unique evolutionary change, or if other animals, including humans, retain this capacity buried somewhere in their genes. If so, it seems that our genes are unable to express this ability, perhaps as part of a tradeoff in acquiring other traits. It is a fertile area of research.
Dedifferentiation is likely to become an important process in the field of regenerative medicine. One extreme example: a lab has been able to turn back the clock and reprogram adult male skin cells into female eggs, a potential milestone in reproductive health. It will be more difficult to control just how far back one wishes to go in the cell's dedifferentiation – part way or all the way back into a stem cell – and then direct it down a different developmental pathway. Yun is optimistic we can learn these tricks from newts.
Senolytics
A growing field of research is using drugs called senolytics to remove senescent cells and slow or even reverse disease of aging.
“Senolytics are great, but senolytics target different types of senescence,” Yun says. “If senescent cells have positive effects in the context of regeneration, of wound healing, then maybe at the beginning of the regeneration process, you may not want to take them out for a little while.”
“If you look at pretty much all biological systems, too little or too much of something can be bad, you have to be in that central zone” and at the proper time, says Atala. “That's true for proteins, sugars, and the drugs that you take. I think the same thing is true for these cells. Why would they be different?”
Our growing understanding that senescence is not a single thing but a variety of things likely means that effective senolytic drugs will not resemble a single sledge hammer but more a carefully manipulated scalpel where some types of senescent cells are removed while others are added. Combinations and timing could be crucial, meaning the difference between regenerating healthy tissue, a scar, or worse.

