Can AI help create “smart borders” between countries?
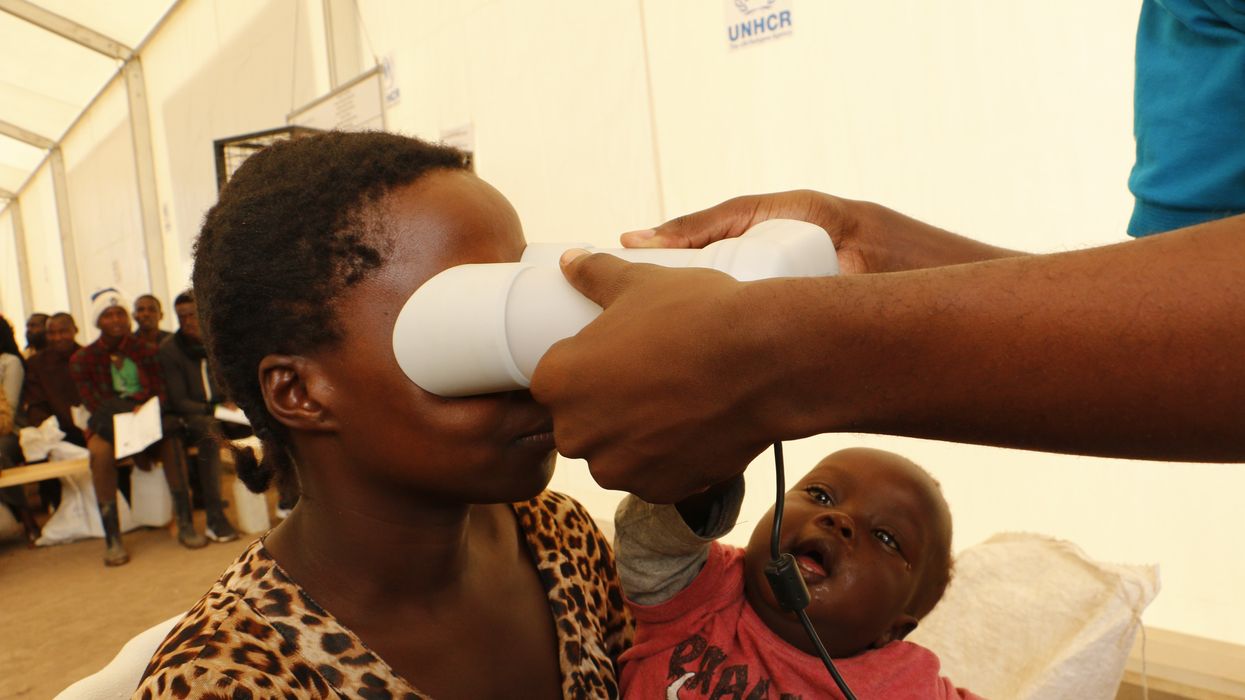
A refugee in Uganda’s Oruchinga settlement uses an iris scan to claim food assistance.
In 2016, border patrols in Greece, Latvia and Hungary received a prototype for an AI-powered lie detector to help screen asylum seekers. The detector, called iBorderCtrl, was funded by the European Commission in hopes to eventually mitigate refugee crises like the one sparked by the Syrian civil war a year prior.
iBorderCtrl, which analyzes micro expressions in the face, received but one slice of the Commission’s €34.9 billion border control and migration management budget. Still in development is the more ambitious EuMigraTool, a predictive AI system that will process internet news and social media posts to estimate not only the number of migrants heading for a particular country, but also the “risks of tensions between migrants and EU citizens.”
Both iBorderCtrl and EuMigraTool are part of a broader trend: the growing digitization of migration-related technologies. Outside of the EU, in refugee camps in Jordan, the United Nations introduced iris scanning software to distribute humanitarian aid, including food and medicine. And in the United States, Customs and Border Protection has attempted to automate its services through an app called CBP One, which both travelers and asylum seekers can use to apply for I-94 forms, the arrival-departure record cards for people who are not U.S. citizens or permanent residents.
According to Koen Leurs, professor of gender, media and migration studies at Utrecht University in the Netherlands, we have arrived at a point where migration management has become so reliant on digital technology that the former can no longer be studied in isolation from the latter. Investigating this reliance for his new book, Digital Migration, Leurs came to the conclusion that applications like those mentioned above are more often than not a double-edged sword, presenting both benefits and drawbacks.
There has been “a huge acceleration” in the way digital technologies “dehumanize people,” says Koen Leurs, professor of gender, media and migration studies at Utrecht University in the Netherlands. Governments treat asylum seekers as test subjects for new inventions, all along the borders of the developed world.
On the one hand, digital technology can make migration management more efficient and less labor intensive, enabling countries to process larger numbers of people in a time when global movement is on the rise due to globalization and political instability. Leurs also discovered that informal knowledge networks such as Informed Immigrant, an online resource that connects migrants to social workers and community organizers, have positively impacted the lives of their users. The same, Leurs notes, is true of platforms like Twitter, Facebook, and WhatsApp, all of which migrants use to stay in touch with each other as well as their families back home. “The emotional support you receive through social media is something we all came to appreciate during the COVID pandemic,” Leurs says. “For refugees, this had already been common knowledge for years.”
On the flipside, automatization of migration management – particularly through the use of AI – has spawned extensive criticism from human rights activists. Sharing their sentiment, Leurs attests that many so-called innovations are making life harder for migrants, not easier. He also says there has been “a huge acceleration” in the way digital technologies “dehumanize people,” and that governments treat asylum seekers as test subjects for new inventions, all along the borders of the developed world.
In Jordan, for example, refugees had to scan their irises in order to collect aid, prompting the question of whether such measures are ethical. Speaking to Reuters, Petra Molnar, a fellow at Harvard University’s Berkman Klein Center for Internet and Society, said that she was troubled by the fact that this experiment was done on marginalized people. “The refugees are guinea pigs,” she said. “Imagine what would happen at your local grocery store if all of a sudden iris scanning became a thing,” she pointed out. “People would be up in arms. But somehow it is OK to do it in a refugee camp.”
Artificial intelligence programs have been scrutinized for their unreliability, their complex processing, thwarted by the race and gender biases picked up from training data. In 2019, a female reporter from The Intercept tested iBorderCtrl and, despite answering all questions truthfully, was accused by the machine of lying four out of 16 times. Had she been waiting at checkpoint on the Greek or Latvian border, she would have been flagged for additional screening – a measure that could jeopardize her chance of entry. Because of its biases, and the negative press that this attracted, iBorderCtrl did not move past its test phase.
While facial recognition caused problems on the European border, it was helpful in Ukraine, where programs like those developed by software company Clearview AI are used to spot Russian spies, identify dead soldiers, and check movement in and out of war zones.
In April 2021, not long after iBorderCtrl was shut down, the European Commission proposed the world’s first-ever legal framework for AI regulation: the Artificial Intelligence Act. The act, which is still being developed, promises to prevent potentially “harmful” AI practices from being used in migration management. In the most recent draft, approved by the European Parliament’s Liberties and Internal Market committees, the ban included emotion recognition systems (like iBorderCtrl), predictive policing systems (like EUMigraTool), and biometric categorization systems (like iris scanners). The act also stipulates that AI must be subject to strict oversight and accountability measures.
While some worry the AI Act is not comprehensive enough, others wonder if it is in fact going too far. Indeed, many proponents of machine learning argue that, by placing a categorical ban on certain systems, governments will thwart the development of potentially useful technology. While facial recognition caused problems on the European border, it was helpful in Ukraine, where programs like those developed by software company Clearview AI are used to spot Russian spies, identify dead soldiers, and check movement in and out of war zones.
Instead of flat-out banning AI, why not strive to make it more reliable? “One of the most compelling arguments against AI is that it is inherently biased,” says Vera Raposo, an assistant professor of law at NOVA University in Lisbon specializing in digital law. “In truth, AI itself is not biased; it becomes biased due to human influence. It seems that complete eradication of biases is unattainable, but mitigation is possible. We can strive to reduce biases by employing more comprehensive and unbiased data in AI training and encompassing a wider range of individuals. We can also work on developing less biased algorithms, although this is challenging given that coders, being human, inherently possess biases of their own.”
AI is most effective when it enhances human performance rather than replacing it.
Accessibility is another obstacle that needs to be overcome. Leurs points out that, in migration management, AI often functions as a “black box” because the migration officers operating it are unable to comprehend its complex decision-making process and thus unable to scrutinize its results. One solution to this problem is to have law enforcement work closely with AI experts. Alternatively, machine learning could be limited to gathering and summarizing information, leaving evaluation of that information to actual people.
Raposo agrees AI is most effective when it enhances human performance rather than replacing it. On the topic of transparency, she does note that making an AI that is both sophisticated and easy to understand is a little bit like having your cake and eating it too. “In numerous domains,” she explains, “we might need to accept a reduced level of explainability in exchange for a high degree of accuracy (assuming we cannot have both).” Using healthcare as an analogy, she adds that “some medications work in ways not fully understood by either doctors or pharma companies, yet persist due to demonstrated efficacy in clinical trials.”
Leurs believes digital technologies used in migration management can be improved through a push for more conscientious research. “Technology is a poison and a medicine for that poison,” he argues, which is why new tech should be developed with its potential applications in mind. “Ethics has become a major concern in recent years. Increasingly, and particularly in the study of forced migration, researchers are posing critical questions like ‘what happens with the data that is gathered?’ and ‘who will this harm?’” In some cases, Leurs thinks, that last question may need to be reversed: we should be thinking about how we can actively disarm oppressive structures. “After all, our work should align with the interests of the communities it is going to affect.”
In this week's Friday Five, new research that could help prevent Alzheimer's. Plus, why you should care about smart senior towns, how to reverse being drunk, money can make you happier if you're this type of person, and personalized anxiety medicine.
The Friday Five covers five stories in research that you may have missed this week. There are plenty of controversies and troubling ethical issues in science – and we get into many of them in our online magazine – but this news roundup focuses on scientific creativity and progress to give you a therapeutic dose of inspiration headed into the weekend.
Here are the promising studies covered in this week's Friday Five, featuring interviews with Dr. Christopher Martens, director of the Delaware Center for Cogntiive Aging Research and professor of kinesiology and applied physiology at the University of Delaware, and Dr. Ilona Matysiak, visiting scholar at Iowa State University and associate professor of sociology at Maria Grzegorzewska University.
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google
- Could this supplement help prevent Alzheimer's?
- Why you should care about smart senior towns
- Here's how to reverse being drunk
- Money can make you happy - if you're this type of person
- Personalized anxiety medicine
As gene therapies and small molecule drugs are being studied in clinical trials, companies increasingly see the value in hiring patients to help explain the potential benefits.
As a child, Wendy Borsari participated in a health study at Boston Children’s Hospital. She was involved because heart disease and sudden cardiac arrest ran in her family as far back as seven generations. When she was 18, however, the study’s doctors told her that she had a perfectly healthy heart and didn’t have to worry.
A couple of years after graduating from college, though, the Boston native began to experience episodes of near fainting. During any sort of strenuous exercise, my blood pressure would drop instead of increasing, she recalls.
She was diagnosed at 24 with hypertrophic cardiomyopathy. Although HCM is a commonly inherited heart disease, Borsari’s case resulted from a rare gene mutation, the MYH7 gene. Her mother had been diagnosed at 27, and Borsari had already lost her grandmother and two maternal uncles to the condition. After her own diagnosis, Borsari spent most of her free time researching the disease and “figuring out how to have this condition and still be the person I wanted to be,” she says.
Then, her son was found to have the genetic mutation at birth and diagnosed with HCM at 15. Her daughter, also diagnosed at birth, later suffered five cardiac arrests.
That changed Borsari’s perspective. She decided to become a patient advocate. “I didn’t want to just be a patient with the condition,” she says. “I wanted to be more involved with the science and the biopharmaceutical industry so I could be active in helping to make it better for other patients.”
She consulted on patient advocacy for a pharmaceutical and two foundations before coming to a company called Tenaya in 2021.
“One of our core values as a company is putting patients first,” says Tenaya's CEO, Faraz Ali. “We thought of no better way to put our money where our mouth is than by bringing in somebody who is affected and whose family is affected by a genetic form of cardiomyopathy to have them make sure we’re incorporating the voice of the patient.”
Biomedical corporations and government research agencies are now incorporating patient advocacy more than ever, says Alice Lara, president and CEO of the Sudden Arrhythmia Death Syndromes Foundation in Salt Lake City, Utah. These organizations have seen the effectiveness of including patient voices to communicate and exemplify the benefits that key academic research institutions have shown in their medical studies.
“From our side of the aisle,” Lara says, “what we know as patient advocacy organizations is that educated patients do a lot better. They have a better course in their therapy and their condition, and understanding the genetics is important because all of our conditions are genetic.”
Founded in 2016, Tenaya is advancing gene therapies and small molecule drugs in clinical trials for both prevalent and rare forms of heart disease, says Ali, the CEO.
The firm's first small molecule, now in a Phase 1 clinical trial, is intended to treat heart failure with preserved ejection fraction, where the amount of blood pumped by the heart is reduced due to the heart chambers becoming weak or stiff. The condition accounts for half or more of all heart failure in the U.S., according to Ali, and is growing quickly because it's closely associated with diabetes. It’s also linked with metabolic syndrome, or a cluster of conditions including high blood pressure, high blood sugar, excess body fat around the waist, and abnormal cholesterol levels.
“We have a novel molecule that is first in class and, to our knowledge, best in class to tackle that, so we’re very excited about the clinical trial,” Ali says.
The first phase of the trial is being performed with healthy participants, rather than people with the disease, to establish safety and tolerability. The researchers can also look for the drug in blood samples, which could tell them whether it's reaching its target. Ali estimates that, if the company can establish safety and that it engages the right parts of the body, it will likely begin dosing patients with the disease in 2024.
Tenaya’s therapy delivers a healthy copy of the gene so that it makes a copy of the protein missing from the patients' hearts because of their mutation. The study will start with adult patients, then pivot potentially to children and even newborns, Ali says, “where there is an even greater unmet need because the disease progresses so fast that they have no options.”
Although this work still has a long way to go, Ali is excited about the potential because the gene therapy achieved positive results in the preclinical mouse trial. This animal trial demonstrated that the treatment reduced enlarged hearts, reversed electrophysiological abnormalities, and improved the functioning of the heart by increasing the ejection fraction after the single-dose of gene therapy. That measurement remained stable to the end of the animals’ lives, roughly 18 months, Ali says.
He’s also energized by the fact that heart disease has “taken a page out of the oncology playbook” by leveraging genetic research to develop more precise and targeted drugs and gene therapies.
“Now we are talking about a potential cure of a disease for which there was no cure and using a very novel concept,” says Melind Desai of the Cleveland Clinic.
Tenaya’s second program focuses on developing a gene therapy to mitigate the leading cause of hypertrophic cardiomyopathy through a specific gene called MYPBC3. The disease affects approximately 600,000 patients in the U.S. This particular genetic form, Ali explains, affects about 115,000 in the U.S. alone, so it is considered a rare disease.
“There are infants who are dying within the first weeks to months of life as a result of this mutation,” he says. “There are also adults who start having symptoms in their 20s, 30s and 40s with early morbidity and mortality.” Tenaya plans to apply before the end of this year to get the FDA’s approval to administer an investigational drug for this disease humans. If approved, the company will begin to dose patients in 2023.
“We now understand the genetics of the heart much better,” he says. “We now understand the leading genetic causes of hypertrophic myopathy, dilated cardiomyopathy and others, so that gives us the ability to take these large populations and stratify them rationally into subpopulations.”
Melind Desai, MD, who directs Cleveland Clinic’s Hypertrophic Cardiomyopathy Center, says that the goal of Tenaya’s second clinical study is to help improve the basic cardiac structure in patients with hypertrophic cardiomyopathy related to the MYPBC3 mutation.
“Now we are talking about a potential cure of a disease for which there was no cure and using a very novel concept,” he says. “So this is an exciting new frontier of therapeutic investigation for MYPBC3 gene-positive patients with a chance for a cure.
Neither of Tenaya’s two therapies address the gene mutation that has affected Borsari and her family. But Ali sees opportunity down the road to develop a gene therapy for her particular gene mutation, since it is the second leading cause of cardiomyopathy. Treating the MYH7 gene is especially challenging because it requires gene editing or silencing, instead of just replacing the gene.

Wendy Borsari was diagnosed at age 24 with a commonly inherited heart disease. She joined Tenaya as a patient advocate in 2021.
Wendy Borsari
“If you add a healthy gene it will produce healthy copies,” Ali explains, “but it won’t stop the bad effects of the mutant protein the gene produces. You can only do that by silencing the gene or editing it out, which is a different, more complicated approach.”
Euan Ashley, professor of medicine and genetics at Stanford University and founding director of its Center for Inherited Cardiovascular Disease, is confident that we will see genetic therapies for heart disease within the next decade.
“We are at this really exciting moment in time where we have diseases that have been under-recognized and undervalued now being attacked by multiple companies with really modern tools,” says Ashley, author of The Genome Odyssey. “Gene therapies are unusual in the sense that they can reverse the cause of the disease, so we have the enticing possibility of actually reversing or maybe even curing these diseases.”
Although no one is doing extensive research into a gene therapy for her particular mutation yet, Borsari remains hopeful, knowing that companies such as Tenaya are moving in that direction.
“I know that’s now on the horizon,” she says. “It’s not just some pipe dream, but will happen hopefully in my lifetime or my kids’ lifetime to help them.”

