Regenerative medicine has come a long way, baby

After a cloned baby sheep, what started as one of the most controversial areas in medicine is now promising to transform it.
The field of regenerative medicine had a shaky start. In 2002, when news spread about the first cloned animal, Dolly the sheep, a raucous debate ensued. Scary headlines and organized opposition groups put pressure on government leaders, who responded by tightening restrictions on this type of research.
Fast forward to today, and regenerative medicine, which focuses on making unhealthy tissues and organs healthy again, is rewriting the code to healing many disorders, though it’s still young enough to be considered nascent. What started as one of the most controversial areas in medicine is now promising to transform it.
Progress in the lab has addressed previous concerns. Back in the early 2000s, some of the most fervent controversy centered around somatic cell nuclear transfer (SCNT), the process used by scientists to produce Dolly. There was fear that this technique could be used in humans, with possibly adverse effects, considering the many medical problems of the animals who had been cloned.
But today, scientists have discovered better approaches with fewer risks. Pioneers in the field are embracing new possibilities for cellular reprogramming, 3D organ printing, AI collaboration, and even growing organs in space. It could bring a new era of personalized medicine for longer, healthier lives - while potentially sparking new controversies.
Engineering tissues from amniotic fluids
Work in regenerative medicine seeks to reverse damage to organs and tissues by culling, modifying and replacing cells in the human body. Scientists in this field reach deep into the mechanisms of diseases and the breakdowns of cells, the little workhorses that perform all life-giving processes. If cells can’t do their jobs, they take whole organs and systems down with them. Regenerative medicine seeks to harness the power of healthy cells derived from stem cells to do the work that can literally restore patients to a state of health—by giving them healthy, functioning tissues and organs.
Modern-day regenerative medicine takes its origin from the 1998 isolation of human embryonic stem cells, first achieved by John Gearhart at Johns Hopkins University. Gearhart isolated the pluripotent cells that can differentiate into virtually every kind of cell in the human body. There was a raging controversy about the use of these cells in research because at that time they came exclusively from early-stage embryos or fetal tissue.
Back then, the highly controversial SCNT cells were the only way to produce genetically matched stem cells to treat patients. Since then, the picture has changed radically because other sources of highly versatile stem cells have been developed. Today, scientists can derive stem cells from amniotic fluid or reprogram patients’ skin cells back to an immature state, so they can differentiate into whatever types of cells the patient needs.
In the context of medical history, the field of regenerative medicine is progressing at a dizzying speed. But for those living with aggressive or chronic illnesses, it can seem that the wheels of medical progress grind slowly.
The ethical debate has been dialed back and, in the last few decades, the field has produced important innovations, spurring the development of whole new FDA processes and categories, says Anthony Atala, a bioengineer and director of the Wake Forest Institute for Regenerative Medicine. Atala and a large team of researchers have pioneered many of the first applications of 3D printed tissues and organs using cells developed from patients or those obtained from amniotic fluid or placentas.
His lab, considered to be the largest devoted to translational regenerative medicine, is currently working with 40 different engineered human tissues. Sixteen of them have been transplanted into patients. That includes skin, bladders, urethras, muscles, kidneys and vaginal organs, to name just a few.
These achievements are made possible by converging disciplines and technologies, such as cell therapies, bioengineering, gene editing, nanotechnology and 3D printing, to create living tissues and organs for human transplants. Atala is currently overseeing clinical trials to test the safety of tissues and organs engineered in the Wake Forest lab, a significant step toward FDA approval.
In the context of medical history, the field of regenerative medicine is progressing at a dizzying speed. But for those living with aggressive or chronic illnesses, it can seem that the wheels of medical progress grind slowly.
“It’s never fast enough,” Atala says. “We want to get new treatments into the clinic faster, but the reality is that you have to dot all your i’s and cross all your t’s—and rightly so, for the sake of patient safety. People want predictions, but you can never predict how much work it will take to go from conceptualization to utilization.”
As a surgeon, he also treats patients and is able to follow transplant recipients. “At the end of the day, the goal is to get these technologies into patients, and working with the patients is a very rewarding experience,” he says. Will the 3D printed organs ever outrun the shortage of donated organs? “That’s the hope,” Atala says, “but this technology won’t eliminate the need for them in our lifetime.”
New methods are out of this world
Jeanne Loring, another pioneer in the field and director of the Center for Regenerative Medicine at Scripps Research Institute in San Diego, says that investment in regenerative medicine is not only paying off, but is leading to truly personalized medicine, one of the holy grails of modern science.
This is because a patient’s own skin cells can be reprogrammed to become replacements for various malfunctioning cells causing incurable diseases, such as diabetes, heart disease, macular degeneration and Parkinson’s. If the cells are obtained from a source other than the patient, they can be rejected by the immune system. This means that patients need lifelong immunosuppression, which isn’t ideal. “With Covid,” says Loring, “I became acutely aware of the dangers of immunosuppression.” Using the patient’s own cells eliminates that problem.
Microgravity conditions make it easier for the cells to form three-dimensional structures, which could more easily lead to the growing of whole organs. In fact, Loring's own cells have been sent to the ISS for study.
Loring has a special interest in neurons, or brain cells that can be developed by manipulating cells found in the skin. She is looking to eventually treat Parkinson’s disease using them. The manipulated cells produce dopamine, the critical hormone or neurotransmitter lacking in the brains of patients. A company she founded plans to start a Phase I clinical trial using cell therapies for Parkinson’s soon, she says.
This is the culmination of many years of basic research on her part, some of it on her own cells. In 2007, Loring had her own cells reprogrammed, so there’s a cell line that carries her DNA. “They’re just like embryonic stem cells, but personal,” she said.
Loring has another special interest—sending immature cells into space to be studied at the International Space Station. There, microgravity conditions make it easier for the cells to form three-dimensional structures, which could more easily lead to the growing of whole organs. In fact, her own cells have been sent to the ISS for study. “My colleagues and I have completed four missions at the space station,” she says. “The last cells came down last August. They were my own cells reprogrammed into pluripotent cells in 2009. No one else can say that,” she adds.
Future controversies and tipping points
Although the original SCNT debate has calmed down, more controversies may arise, Loring thinks.
One of them could concern growing synthetic embryos. The embryos are ultimately derived from embryonic stem cells, and it’s not clear to what stage these embryos can or will be grown in an artificial uterus—another recent invention. The science, so far done only in animals, is still new and has not been widely publicized but, eventually, “People will notice the production of synthetic embryos and growing them in an artificial uterus,” Loring says. It’s likely to incite many of the same reactions as the use of embryonic stem cells.
Bernard Siegel, the founder and director of the Regenerative Medicine Foundation and executive director of the newly formed Healthspan Action Coalition (HSAC), believes that stem cell science is rapidly approaching tipping point and changing all of medical science. (For disclosure, I do consulting work for HSAC). Siegel says that regenerative medicine has become a new pillar of medicine that has recently been fast-tracked by new technology.
Artificial intelligence is speeding up discoveries and the convergence of key disciplines, as demonstrated in Atala’s lab, which is creating complex new medical products that replace the body’s natural parts. Just as importantly, those parts are genetically matched and pose no risk of rejection.
These new technologies must be regulated, which can be a challenge, Siegel notes. “Cell therapies represent a challenge to the existing regulatory structure, including payment, reimbursement and infrastructure issues that 20 years ago, didn’t exist.” Now the FDA and other agencies are faced with this revolution, and they’re just beginning to adapt.
Siegel cited the 2021 FDA Modernization Act as a major step. The Act allows drug developers to use alternatives to animal testing in investigating the safety and efficacy of new compounds, loosening the agency’s requirement for extensive animal testing before a new drug can move into clinical trials. The Act is a recognition of the profound effect that cultured human cells are having on research. Being able to test drugs using actual human cells promises to be far safer and more accurate in predicting how they will act in the human body, and could accelerate drug development.
Siegel, a longtime veteran and founding father of several health advocacy organizations, believes this work helped bring cell therapies to people sooner rather than later. His new focus, through the HSAC, is to leverage regenerative medicine into extending not just the lifespan but the worldwide human healthspan, the period of life lived with health and vigor. “When you look at the HSAC as a tree,” asks Siegel, “what are the roots of that tree? Stem cell science and the huge ecosystem it has created.” The study of human aging is another root to the tree that has potential to lengthen healthspans.
The revolutionary science underlying the extension of the healthspan needs to be available to the whole world, Siegel says. “We need to take all these roots and come up with a way to improve the life of all mankind,” he says. “Everyone should be able to take advantage of this promising new world.”
A Mother-and-Daughter Team Have Developed What May Be the World’s First Alzheimer’s Vaccine
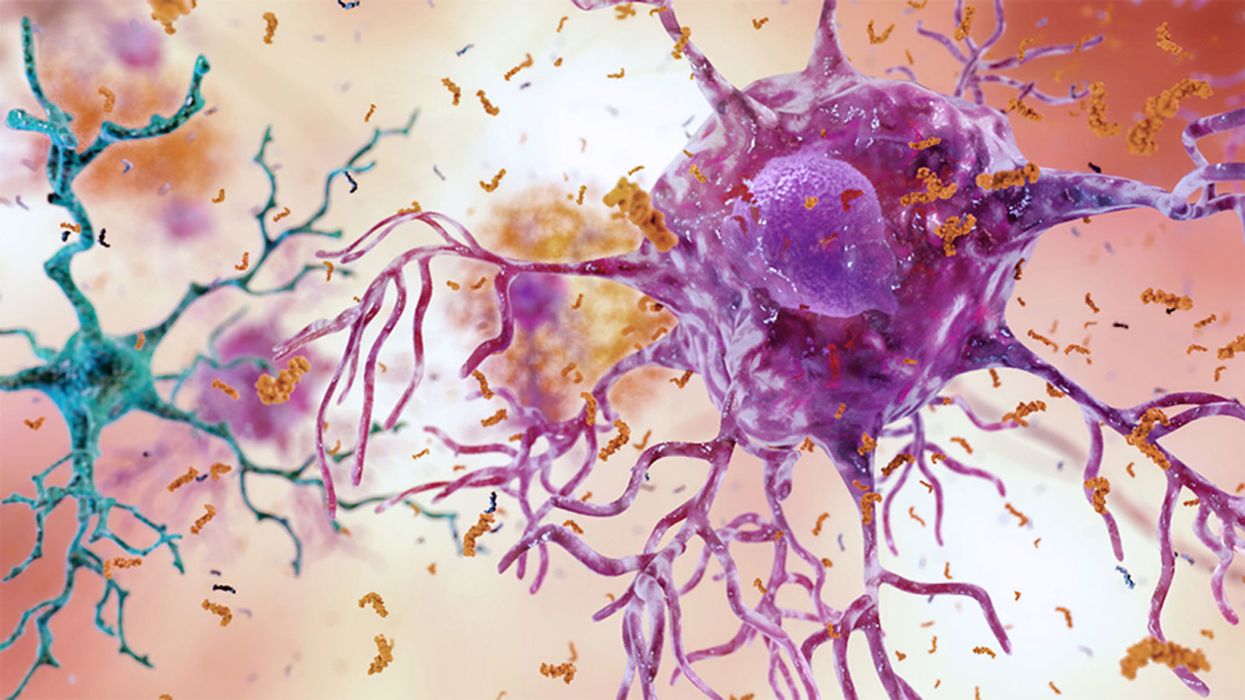
Brain inflammation from Alzheimer's disease.
Alzheimer's is a terrible disease that robs a person of their personality and memory before eventually leading to death. It's the sixth-largest killer in the U.S. and, currently, there are 5.8 million Americans living with the disease.
Wang's vaccine is a significant improvement over previous attempts because it can attack the Alzheimer's protein without creating any adverse side effects.
It devastates people and families and it's estimated that Alzheimer's and other forms of dementia will cost the U.S. $290 billion dollars this year alone. It's estimated that it will become a trillion-dollar-a-year disease by 2050.
There have been over 200 unsuccessful attempts to find a cure for the disease and the clinical trial termination rate is 98 percent.
Alzheimer's is caused by plaque deposits that develop in brain tissue that become toxic to brain cells. One of the major hurdles to finding a cure for the disease is that it's impossible to clear out the deposits from the tissue. So scientists have turned their attention to early detection and prevention.
One very encouraging development has come out of the work done by Dr. Chang Yi Wang, PhD. Wang is a prolific bio-inventor; one of her biggest successes is developing a foot-and-mouth vaccine for pigs that has been administered more than three billion times.

Mei Mei Hu
Brainstorm Health / Flickr.
In January, United Neuroscience, a biotech company founded by Yi, her daughter Mei Mei Hu, and son-in-law, Louis Reese, announced the first results from a phase IIa clinical trial on UB-311, an Alzheimer's vaccine.
The vaccine has synthetic versions of amino acid chains that trigger antibodies to attack Alzheimer's protein the blood. Wang's vaccine is a significant improvement over previous attempts because it can attack the Alzheimer's protein without creating any adverse side effects.
"We were able to generate some antibodies in all patients, which is unusual for vaccines," Yi told Wired. "We're talking about almost a 100 percent response rate. So far, we have seen an improvement in three out of three measurements of cognitive performance for patients with mild Alzheimer's disease."
The researchers also claim it can delay the onset of the disease by five years. While this would be a godsend for people with the disease and their families, according to Elle, it could also save Medicare and Medicaid more than $220 billion.
"You'd want to see larger numbers, but this looks like a beneficial treatment," James Brown, director of the Aston University Research Centre for Healthy Ageing, told Wired. "This looks like a silver bullet that can arrest or improve symptoms and, if it passes the next phase, it could be the best chance we've got."
"A word of caution is that it's a small study," says Drew Holzapfel, acting president of the nonprofit UsAgainstAlzheimer's, said according to Elle. "But the initial data is compelling."
The company is now working on its next clinical trial of the vaccine and while hopes are high, so is the pressure. The company has already invested $100 million developing its vaccine platform. According to Reese, the company's ultimate goal is to create a host of vaccines that will be administered to protect people from chronic illness.
"We have a 50-year vision -- to immuno-sculpt people against chronic illness and chronic aging with vaccines as prolific as vaccines for infectious diseases," he told Elle.
[Editor's Note: This article was originally published by Upworthy here and has been republished with permission.]
Turning Algae Into Environmentally Friendly Fuel Just Got Faster and Smarter
Algae in the Mediterranean sea.
Was your favorite beach closed this summer? Algae blooms are becoming increasingly the reason to blame and, as the climate heats up, scientists say we can expect more of the warm water-loving blue-green algae to grow.
"We have removed a significant development barrier to make algal biofuel production more efficient and smarter."
Oddly enough, the pesky growth could help fuel our carbon-friendly options.
This year, the University of Utah scientists discovered a faster way to turn algae into fuel. Algae is filled with lipids that we can feed our energy-hungry diesel engines. The problem is extracting the lipids, which usually requires more energy to transform than the actual energy we'd get – not achieving what scientists call "energy parity."
But now, the University of Utah team has discovered a new mix that is more efficient and much faster. We can now extract more power from algae with less waste materials after the fact. Paper co-author Dr. Leonard Pease says, "We have removed a significant development barrier to make algal biofuel production more efficient and smarter. Our method puts us much closer to creating biofuels energy parity than we were before."
Next Up
Algae has a lot going for it as an alternative fuel source. It grows fast and easily, absorbs carbon dioxide, does not compete with food crops for land, and could produce up to 60 times more oil than standard land-based energy crops, according to the U.S. Department of Energy. Yet the costs of algal biofuel production are still expensive for now.
According to Science Daily, only about five percent of total primary energy use in the United States came from algae and other biomass forms. By making the process more efficient, America and other nations could potentially begin relying on more plentiful resources – which, ironically, are more common now because of climate change.
Algae fuel efficiency is already a proven concept. A decade ago, Continental Airlines completed a 90-minute Boeing 737-800 flight with one engine split between biofuel and aircraft fuel. The biofuel was straight from algae. (Other flights were done based on nut fuel and other alternative sources.) The commercial airplane required no modification to the engine and the biofuel itself exceeded the standards of traditional jet fuel.
The problem, as noted at the time, is that biofuels derived from algae had yet to be proven as "commercially competitive."
The University of Utah's discovery could mean cheaper processing. At this point, it is less about if it works and more about if it is a practical alternative.
However, it's unclear how long it will take for algae to become more mainstream, if ever.
Open Questions
Higher efficiency and simpler transformations could mean lower prices and more business access. However, it's unclear how long it will take for algae to become more mainstream, if ever. The algae biofuel worked great for a relatively sophisticated Boeing 737 engine, but your family car, the cross-country delivery trucks and other less powerful machines may need to be modified – and that means the industry-at-large would have to revise their products in order to support the change.
Future-focused groups are already looking at how algae can fuel our space programs, especially if it is more renewable, safe and, potentially, cheaper than our traditional fuel choices. But first, it is worth waiting and seeing if corporations and, later, citizens are willing to take the plunge.