Regulation Too Often Shackles the Hands of Innovators

[Editor's Note: Our Big Moral Question this month is, "Do government regulations help or hurt the goal of responsible and timely scientific innovation?"]
After biomedical scientists demonstrated that they could make dangerous viruses like influenza even more dangerous, the National Institutes of Health (NIH) implemented a three-year moratorium on funding such research. But a couple of months ago, in December, the moratorium was lifted, and a tight set of rules were put in its place, such as a mandate for oversight panels.
"The sort of person who thinks like a bureaucratic regulator isn't the sort of person who thinks like a scientist."
The prospect of engineering a deadly pandemic virus in a laboratory suggests that only a fool would wish away government regulation entirely.
However, as a whole, regulation has done more harm than good in the arena of scientific innovation. The reason is that the sort of person who thinks like a bureaucratic regulator isn't the sort of person who thinks like a scientist. The sad fact of the matter is that those most interested in the regulatory process tend to be motivated by politics and ideology rather than scientific inquiry and technological progress.
Consider genetically engineered crops and animals, for instance. Beyond any reasonable doubt, data consistently have shown them to be safe, yet they are routinely held in regulatory limbo. For instance, it took 20 years for the AquAdvantage salmon, which grows faster than ordinary salmon, to gain approval from the FDA. What investor in his right mind would fund an entrepreneurial scientist who wishes to create genetically engineered consumer goods when he is assured that any such product could be subjected to two decades of arbitrary and pointless bureaucratic scrutiny?
Other well-intentioned regulations have created enormous problems for society. Medicine costs too much. One reason is that there is no international competition in the U.S. marketplace because it is nearly impossible to import drugs from other countries. The FDA's overcautious attitude toward approving new medications has ushered in a grassroots "right-to-try" movement, in which terminal patients are demanding access to potentially life-saving (but also potentially dangerous) treatments that are not yet federally approved. The FDA's sluggishness in approving generics also allowed the notorious former hedge fund manager Martin Shkreli to jack up the price of a drug for HIV patients because there were no competitors on the market. Thankfully, the FDA and politicians are now aware of these self-inflicted problems and are proposing possible solutions.
"Other well-intentioned regulations have created enormous problems for society."
The regulatory process itself drags on far too long and consists of procedural farces, none more so than public hearings and the solicitation of public comments. Hearings are often dominated by activists who are more concerned with theatrics and making the front page of a newspaper rather than contributing meaningfully to the scientific debate.
It is frankly absurd to believe that scientifically untrained laypeople have anything substantive to say on matters like biomedical regulation. The generals at the Pentagon quite rightly do not seek the public's council before they draw up battlefield plans, so why should scientists be subjected to an unjustifiable level of public scrutiny? Besides, there is a good chance that a substantial proportion of feedback is fake, anyway: A Wall Street Journal investigation uncovered that thousands of posts on federal websites seeking public comment on topics like net neutrality are fraudulent.
In other cases, out-of-date regulations remain on the books, holding back progress. For more than 20 years, the Dickey-Wicker Amendment has tied the hands of the NIH, essentially preventing it from funding any research that must first create human embryos or derive new embryonic stem cell lines. This seriously impedes progress in regenerative medicine and dampens the potential revolutionary potential of CRISPR, a genome editing tool that could someday be used in adult gene therapy or to "fix" unhealthy human embryos.
"Regulators and especially politicians give the false impression that any new scientific innovation should be made perfectly safe before it is allowed on the market."
Biomedicine isn't the only science to suffer at the hands of regulators. For years, the Nuclear Regulatory Commission (NRC) – an organization ostensibly concerned about nuclear safety – instead has played politics with nuclear power, particularly over a proposed waste storage facility at Yucca Mountain. Going all the way back to the Reagan administration, Yucca has been subjected to partisan assaults, culminating in the Obama administration's mothballing the project. Under the Trump administration, the NRC is once again reconsidering its future.
Perhaps the biggest problem that results from overregulation is a change in the culture. Regulators and especially politicians give the false impression that any new scientific innovation should be made perfectly safe before it is allowed on the market. This notion is known as the precautionary principle, and it is the law in the European Union. The precautionary principle is a form of technological timidity that is partially to blame for Europe's lagging behind America in groundbreaking research.
Besides, perfect safety is an impossible goal. Nothing in life is perfectly safe. The same people who drive to Whole Foods to avoid GMOs and synthetic pesticides seem not to care that automobiles kill 30,000 Americans every single year.
Government regulation is necessary because people rightfully expect a safe place to work and live. However, charlatans and lawbreakers will always exist, no matter how many new rules are added. The proliferation of safety regulations, therefore, often results in increasing the burden on innovators without any concomitant increase in safety. Like an invasive weed, government regulation has spread far beyond its proper place in the ecosystem. It's time for a weedkiller.
[Ed. Note: Check out the opposite viewpoint here, and follow LeapsMag on social media to share your perspective.]
A robot server, controlled remotely by a disabled worker, delivers drinks to patrons at the DAWN cafe in Tokyo.
A sleek, four-foot tall white robot glides across a cafe storefront in Tokyo’s Nihonbashi district, holding a two-tiered serving tray full of tea sandwiches and pastries. The cafe’s patrons smile and say thanks as they take the tray—but it’s not the robot they’re thanking. Instead, the patrons are talking to the person controlling the robot—a restaurant employee who operates the avatar from the comfort of their home.
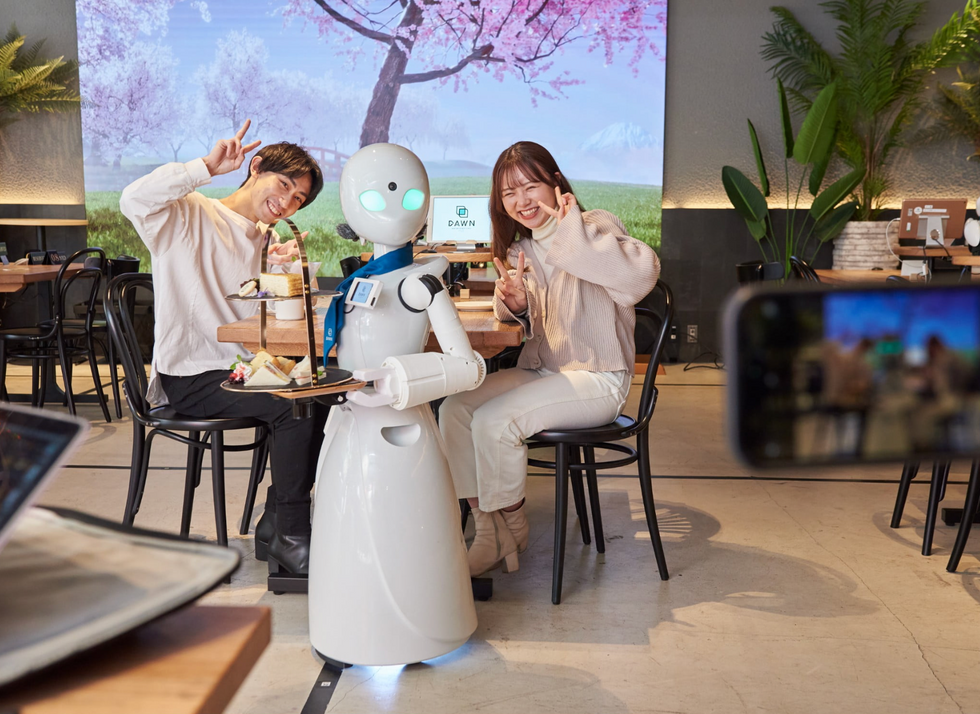
It’s a typical scene at DAWN, short for Diverse Avatar Working Network—a cafe that launched in Tokyo six years ago as an experimental pop-up and quickly became an overnight success. Today, the cafe is a permanent fixture in Nihonbashi, staffing roughly 60 remote workers who control the robots remotely and communicate to customers via a built-in microphone.
More than just a creative idea, however, DAWN is being hailed as a life-changing opportunity. The workers who control the robots remotely (known as “pilots”) all have disabilities that limit their ability to move around freely and travel outside their homes. Worldwide, an estimated 16 percent of the global population lives with a significant disability—and according to the World Health Organization, these disabilities give rise to other problems, such as exclusion from education, unemployment, and poverty.
These are all problems that Kentaro Yoshifuji, founder and CEO of Ory Laboratory, which supplies the robot servers at DAWN, is looking to correct. Yoshifuji, who was bedridden for several years in high school due to an undisclosed health problem, launched the company to help enable people who are house-bound or bedridden to more fully participate in society, as well as end the loneliness, isolation, and feelings of worthlessness that can sometimes go hand-in-hand with being disabled.
“It’s heartbreaking to think that [people with disabilities] feel they are a burden to society, or that they fear their families suffer by caring for them,” said Yoshifuji in an interview in 2020. “We are dedicating ourselves to providing workable, technology-based solutions. That is our purpose.”
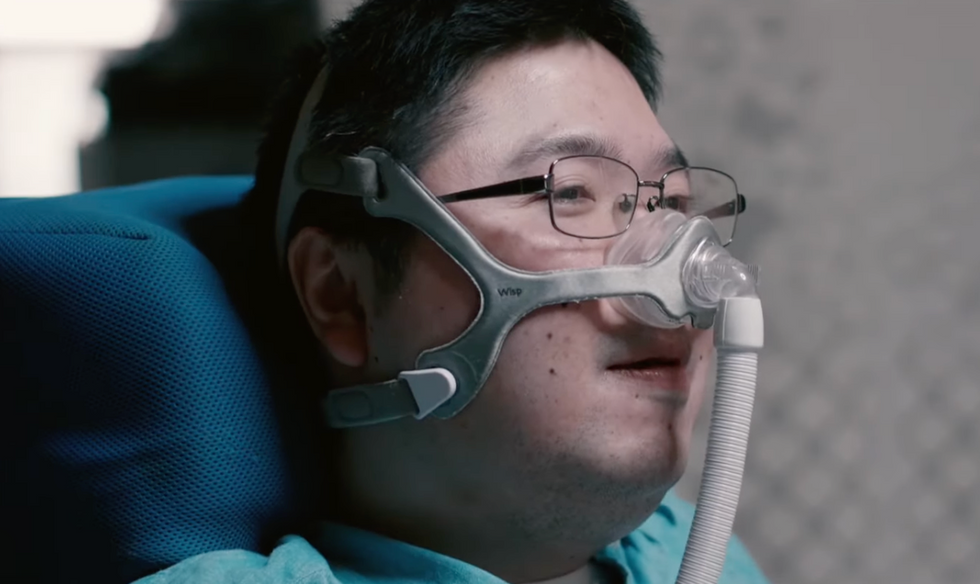
Shota, Kuwahara, a DAWN employee with muscular dystrophy, agrees. "There are many difficulties in my daily life, but I believe my life has a purpose and is not being wasted," he says. "Being useful, able to help other people, even feeling needed by others, is so motivational."
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.

