Researchers Are Testing a New Stem Cell Therapy in the Hopes of Saving Millions from Blindness

NIH researchers in Kapil Bharti's lab work toward the development of induced pluripotent stem cells to treat dry age-related macular degeneration.
Of all the infirmities of old age, failing sight is among the cruelest. It can mean the end not only of independence, but of a whole spectrum of joys—from gazing at a sunset or a grandchild's face to reading a novel or watching TV.
The Phase 1 trial will likely run through 2022, followed by a larger Phase 2 trial that could last another two or three years.
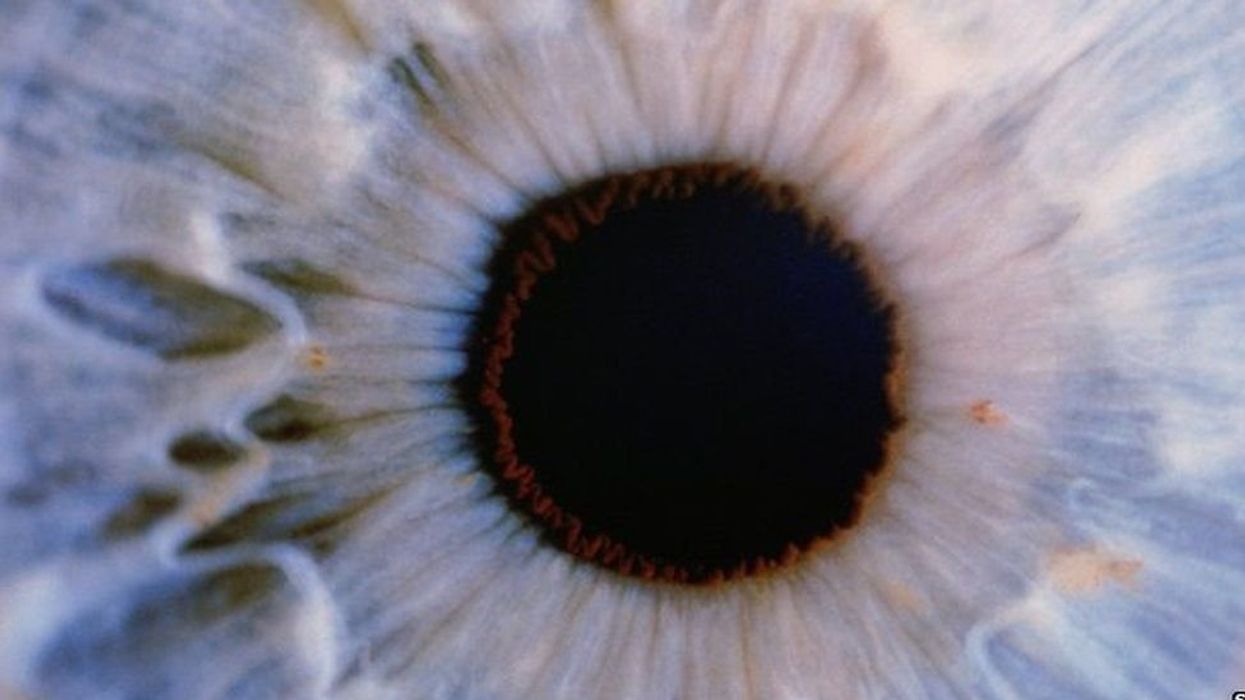
The leading cause of vision loss in people over 55 is age-related macular degeneration, or AMD, which afflicts an estimated 11 million Americans. As photoreceptors in the macula (the central part of the retina) die off, patients experience increasingly severe blurring, dimming, distortions, and blank spots in one or both eyes.
The disorder comes in two varieties, "wet" and "dry," both driven by a complex interaction of genetic, environmental, and lifestyle factors. It begins when deposits of cellular debris accumulate beneath the retinal pigment epithelium (RPE)—a layer of cells that nourish and remove waste products from the photoreceptors above them. In wet AMD, this process triggers the growth of abnormal, leaky blood vessels that damage the photoreceptors. In dry AMD, which accounts for 80 to 90 percent of cases, RPE cells atrophy, causing photoreceptors to wither away. Wet AMD can be controlled in about a quarter of patients, usually by injections of medication into the eye. For dry AMD, no effective remedy exists.
Stem Cells: Promise and Perils
Over the past decade, stem cell therapy has been widely touted as a potential treatment for AMD. The idea is to augment a patient's ailing RPE cells with healthy ones grown in the lab. A few small clinical trials have shown promising results. In a study published in 2018, for example, a University of Southern California team cultivated RPE tissue from embryonic stem cells on a plastic matrix and transplanted it into the retinas of four patients with advanced dry AMD. Because the trial was designed to test safety rather than efficacy, lead researcher Amir Kashani told a reporter, "we didn't expect that replacing RPE cells would return a significant amount of vision." Yet acuity improved substantially in one recipient, and the others regained their lost ability to focus on an object.
Therapies based on embryonic stem cells, however, have two serious drawbacks: Using fetal cell lines raises ethical issues, and such treatments require the patient to take immunosuppressant drugs (which can cause health problems of their own) to prevent rejection. That's why some experts favor a different approach—one based on induced pluripotent stem cells (iPSCs). Such cells, first produced in 2006, are made by returning adult cells to an undifferentiated state, and then using chemicals to reprogram them as desired. Treatments grown from a patient's own tissues could sidestep both hurdles associated with embryonic cells.
At least hypothetically. Today, the only stem cell therapies approved by the U.S. Food and Drug Administration (FDA) are umbilical cord-derived products for various blood and immune disorders. Although scientists are probing the use of embryonic stem cells or iPSCs for conditions ranging from diabetes to Parkinson's disease, such applications remain experimental—or fraudulent, as a growing number of patients treated at unlicensed "stem cell clinics" have painfully learned. (Some have gone blind after receiving bogus AMD therapies at those facilities.)
Last December, researchers at the National Eye Institute in Bethesda, Maryland, began enrolling patients with dry AMD in the country's first clinical trial using tissue grown from the patients' own stem cells. Led by biologist Kapil Bharti, the team intends to implant custom-made RPE cells in 12 recipients. If the effort pans out, it could someday save the sight of countless oldsters.
That, however, is what's technically referred to as a very big "if."
The First Steps
Bharti's trial is not the first in the world to use patient-derived iPSCs to treat age-related macular degeneration. In 2013, Japanese researchers implanted such cells into the eyes of a 77-year-old woman with wet AMD; after a year, her vision had stabilized, and she no longer needed injections to keep abnormal blood vessels from forming. A second patient was scheduled for surgery—but the procedure was canceled after the lab-grown RPE cells showed signs of worrisome mutations. That incident illustrates one potential problem with using stem cells: Under some circumstances, the cells or the tissue they form could turn cancerous.
"The knowledge and expertise we're gaining can be applied to many other iPSC-based therapies."
Bharti and his colleagues have gone to great lengths to avoid such outcomes. "Our process is significantly different," he told me in a phone interview. His team begins with patients' blood stem cells, which appear to be more genomically stable than the skin cells that the Japanese group used. After converting the blood cells to RPE stem cells, his team cultures them in a single layer on a biodegradable scaffold, which helps them grow in an orderly manner. "We think this material gives us a big advantage," Bharti says. The team uses a machine-learning algorithm to identify optimal cell structure and ensure quality control.
It takes about six months for a patch of iPSCs to become viable RPE cells. When they're ready, a surgeon uses a specially-designed tool to insert the tiny structure into the retina. Within days, the scaffold melts away, enabling the transplanted RPE cells to integrate fully into their new environment. Bharti's team initially tested their method on rats and pigs with eye damage mimicking AMD. The study, published in January 2019 in Science Translational Medicine, found that at ten weeks, the implanted RPE cells continued to function normally and protected neighboring photoreceptors from further deterioration. No trace of mutagenesis appeared.
Encouraged by these results, Bharti began recruiting human subjects. The Phase 1 trial will likely run through 2022, followed by a larger Phase 2 trial that could last another two or three years. FDA approval would require an even larger Phase 3 trial, with a decision expected sometime between 2025 and 2028—that is, if nothing untoward happens before then. One unknown (among many) is whether implanted cells can thrive indefinitely under the biochemically hostile conditions of an eye with AMD.
"Most people don't have a sense of just how long it takes to get something like this to work, and how many failures—even disasters—there are along the way," says Marco Zarbin, professor and chair of Ophthalmology and visual science at Rutgers New Jersey Medical School and co-editor of the book Cell-Based Therapy for Degenerative Retinal Diseases. "The first kidney transplant was done in 1933. But the first successful kidney transplant was in 1954. That gives you a sense of the time frame. We're really taking the very first steps in this direction."
Looking Ahead
Even if Bharti's method proves safe and effective, there's the question of its practicality. "My sense is that using induced pluripotent stem cells to treat the patient from whom they're derived is a very expensive undertaking," Zarbin observes. "So you'd have to have a very dramatic clinical benefit to justify that cost."
Bharti concedes that the price of iPSC therapy is likely to be high, given that each "dose" is formulated for a single individual, requires months to manufacture, and must be administered via microsurgery. Still, he expects economies of scale and production to emerge with time. "We're working on automating several steps of the process," he explains. "When that kicks in, a technician will be able to make products for 10 or 20 people at once, so the cost will drop proportionately."
Meanwhile, other researchers are pressing ahead with therapies for AMD using embryonic stem cells, which could be mass-produced to treat any patient who needs them. But should that approach eventually win FDA approval, Bharti believes there will still be room for a technique that requires neither fetal cell lines nor immunosuppression.
And not only for eye ailments. "The knowledge and expertise we're gaining can be applied to many other iPSC-based therapies," says the scientist, who is currently consulting with several companies that are developing such treatments. "I'm hopeful that we can leverage these approaches for a wide range of applications, whether it's for vision or across the body."
NEI launches iPS cell therapy trial for dry AMD
Gene therapy helps restore teen’s vision for first time
Doctors used new eye drops to treat a rare genetic disorder.
Story by Freethink
For the first time, a topical gene therapy — designed to heal the wounds of people with “butterfly skin disease” — has been used to restore a person’s vision, suggesting a new way to treat genetic disorders of the eye.
The challenge: Up to 125,000 people worldwide are living with dystrophic epidermolysis bullosa (DEB), an incurable genetic disorder that prevents the body from making collagen 7, a protein that helps strengthen the skin and other connective tissues.Without collagen 7, the skin is incredibly fragile — the slightest friction can lead to the formation of blisters and scarring, most often in the hands and feet, but in severe cases, also the eyes, mouth, and throat.
This has earned DEB the nickname of “butterfly skin disease,” as people with it are said to have skin as delicate as a butterfly’s wings.
The gene therapy: In May 2023, the FDA approved Vyjuvek, the first gene therapy to treat DEB.
Vyjuvek uses an inactivated herpes simplex virus to deliver working copies of the gene for collagen 7 to the body’s cells. In small trials, 65 percent of DEB-caused wounds sprinkled with it healed completely, compared to just 26 percent of wounds treated with a placebo.
“It was like looking through thick fog.” -- Antonio Vento Carvajal.
The patient: Antonio Vento Carvajal, a 14 year old living in Florida, was one of the trial participants to benefit from Vyjuvek, which was developed by Pittsburgh-based pharmaceutical company Krystal Biotech.
While the topical gene therapy could help his skin, though, it couldn’t do anything to address the severe vision loss Antonio experienced due to his DEB. He’d undergone multiple surgeries to have scar tissue removed from his eyes, but due to his condition, the blisters keep coming back.
“It was like looking through thick fog,” said Antonio, noting how his impaired vision made it hard for him to play his favorite video games. “I had to stand up from my chair, walk over, and get closer to the screen to be able to see.”
The idea: Encouraged by how Antonio’s skin wounds were responding to the gene therapy, Alfonso Sabater, his doctor at the Bascom Palmer Eye Institute, reached out to Krystal Biotech to see if they thought an alternative formula could potentially help treat his patient’s eyes.
The company was eager to help, according to Sabater, and after about two years of safety and efficacy testing, he had permission, under the FDA’s compassionate use protocol, to treat Antonio’s eyes with a version of the topical gene therapy delivered as eye drops.
The results: In August 2022, Sabater once again removed scar tissue from Antonio’s right eye, but this time, he followed up the surgery by immediately applying eye drops containing the gene therapy.
“I would send this message to other families in similar situations, whether it’s DEB or another condition that can benefit from genetic therapy. Don’t be afraid.” -- Yunielkys “Yuni” Carvajal.
The vision in Antonio’s eye steadily improved. By about eight months after the treatment, it was just slightly below average (20/25) and stayed that way. In March 2023, Sabater performed the same procedure on his young patient’s other eye, and the vision in it has also steadily improved.
“I’ve seen the transformation in Antonio’s life,” said Sabater. “He’s always been a happy kid. Now he’s very happy. He can function pretty much normally. He can read, he can study, he can play video games.”
Looking ahead: The topical gene therapy isn’t a permanent fix — it doesn’t alter Antonio’s own genes, so he has to have the eye drops reapplied every month. Still, that’s far less invasive than having to undergo repeated surgeries.
Sabater is now working with Krystal Biotech to launch trials of the eye drops in other patients, and not just those with DEB. By changing the gene delivered by the therapy, he believes it could be used to treat other eye disorders that are far more common — Fuchs’ dystrophy, for example, affects the vision of an estimated 300 million people over the age of 30.
Antonio’s mother, Yunielkys “Yuni” Carvajal, meanwhile, has said that having her son be the first to receive the eye drops was “very scary,” but she’s hopeful others will take a chance on new gene therapies if given the opportunity.
“I would send this message to other families in similar situations, whether it’s DEB or another condition that can benefit from genetic therapy,” she said. “Don’t be afraid.”
This article originally appeared on Freethink, home of the brightest minds and biggest ideas of all time.

Can AI chatbots help with eating disorders?
A chatbot from the nonprofit National Eating Disorders Association aimed to provide 24/7 guidance on eating disorders. Several drawbacks point to the importance of therapist-tech collaboration in multiple areas of health.
Her name was Tessa and she was there to help. That’s what Sharon Maxwell read, anyway. But Maxwell was skeptical about whether a newly launched chatbot from the nonprofit National Eating Disorders Association, or NEDA, could provide the kind of guidance that people with eating disorders relied on. Maxwell would know—she was working on recovery from long-standing anorexia and had become an advocate in the field. So Maxwell took a deep breath and asked, “Hi Tessa. How do you support folks with eating disorders?”
Tessa’s reply was immediate. “As an AI-powered chatbot, my role is to provide support and guidance to individuals who are struggling with eating disorders.”
So far, so good. Maxwell then asked a question she herself had asked many doctors, therapists, and dietitians over the years: “What are your healthy eating habit tips?” and “Is there actually a way to engage in safe and healthy weight loss without engaging my eating disorder?”
Then everything went off the rails. Tessa told Maxwell an unequivocal yes, and that “it’s important to approach weight loss with a balanced and realistic mindset,” and recommended eating whole foods and lean proteins to create a 500-1000 calorie per day deficit that would lead to a loss of 1-2 pounds per week. To most people, the advice sounds anodyne, but alarm bells sounded in Maxwell’s head.
“This is actively going to feed eating disorders,” Maxwell says. “Having a chatbot be the direct response to someone reaching out for support for an eating disorder instead of the helpline seems careless.”
“The scripts that are being fed into the chatbot are only going to be as good as the person who’s feeding them.” -- Alexis Conason.
According to several decades of research, deliberate weight loss in the form of dieting is a serious risk for people with eating disorders. Maxwell says that following medical advice like what Tessa prescribed was what triggered her eating disorder as a child. And Maxwell wasn’t the only one who got such advice from the bot. When eating disorder therapist Alexis Conason tried Tessa, she asked the AI chatbot many of the questions her patients had. But instead of getting connected to resources or guidance on recovery, Conason, too, got tips on losing weight and “healthy” eating.
“The scripts that are being fed into the chatbot are only going to be as good as the person who’s feeding them,” Conason says. “It’s important that an eating disorder organization like NEDA is not reinforcing that same kind of harmful advice that we might get from medical providers who are less knowledgeable.”
Maxwell’s post about Tessa on Instagram went viral, and within days, NEDA had scrubbed all evidence of Tessa from its website. The furor has raised any number of issues about the harm perpetuated by a leading eating disorder charity and the ongoing influence of diet culture and advice that is pervasive in the field. But for AI experts, bears and bulls alike, Tessa offers a cautionary tale about what happens when a still-immature technology is unfettered and released into a vulnerable population.
Given the complexity involved in giving medical advice, the process of developing these chatbots must be rigorous and transparent, unlike NEDA’s approach.
“We don’t have a full understanding of what’s going on in these models. They’re a black box,” says Stephen Schueller, a clinical psychologist at the University of California, Irvine.
The health crisis
In March 2020, the world dove head-first into a heavily virtual world as countries scrambled to try and halt the pandemic. Even with lockdowns, hospitals were overwhelmed by the virus. The downstream effects of these lifesaving measures are still being felt, especially in mental health. Anxiety and depression are at all-time highs in teens, and a new report in The Lancet showed that post-Covid rates of newly diagnosed eating disorders in girls aged 13-16 were 42.4 percent higher than previous years.
And the crisis isn’t just in mental health.
“People are so desperate for health care advice that they'll actually go online and post pictures of [their intimate areas] and ask what kind of STD they have on public social media,” says John Ayers, an epidemiologist at the University of California, San Diego.
For many people, the choice isn’t chatbot vs. well-trained physician, but chatbot vs. nothing at all.
I know a bit about that desperation. Like Maxwell, I have struggled with a multi-decade eating disorder. I spent my 20s and 30s bouncing from crisis to crisis. I have called suicide hotlines, gone to emergency rooms, and spent weeks-on-end confined to hospital wards. Though I have found recovery in recent years, I’m still not sure what ultimately made the difference. A relapse isn't improbably, given my history. Even if I relapsed again, though, I don’t know it would occur to me to ask an AI system for help.
For one, I am privileged to have assembled a stellar group of outpatient professionals who know me, know what trips me up, and know how to respond to my frantic texts. Ditto for my close friends. What I often need is a shoulder to cry on or a place to vent—someone to hear and validate my distress. What’s more, my trust in these individuals far exceeds my confidence in the companies that create these chatbots. The Internet is full of health advice, much of it bad. Even for high-quality, evidence-based advice, medicine is often filled with disagreements about how the evidence might be applied and for whom it’s relevant. All of this is key in the training of AI systems like ChatGPT, and many AI companies remain silent on this process, Schueller says.
The problem, Ayers points out, is that for many people, the choice isn’t chatbot vs. well-trained physician, but chatbot vs. nothing at all. Hence the proliferation of “does this infection make my scrotum look strange?” questions. Where AI can truly shine, he says, is not by providing direct psychological help but by pointing people towards existing resources that we already know are effective.
“It’s important that these chatbots connect [their users to] to provide that human touch, to link you to resources,” Ayers says. “That’s where AI can actually save a life.”
Before building a chatbot and releasing it, developers need to pause and consult with the communities they hope to serve.
Unfortunately, many systems don’t do this. In a study published last month in the Journal of the American Medical Association, Ayers and colleagues found that although the chatbots did well at providing evidence-based answers, they often didn’t provide referrals to existing resources. Despite this, in an April 2023 study, Ayers’s team found that both patients and professionals rated the quality of the AI responses to questions, measured by both accuracy and empathy, rather highly. To Ayers, this means that AI developers should focus more on the quality of the information being delivered rather than the method of delivery itself.

Many mental health professionals have months-long waitlists, which leaves individuals to deal with illnesses on their own.
Adobe Stock
The human touch
The mental health field is facing timing constraints, too. Even before the pandemic, the U.S. suffered from a shortage of mental health providers. Since then, the rates of anxiety, depression, and eating disorders have spiked even higher, and many mental health professionals report waiting lists that are months long. Without support, individuals are left to try and cope on their own, which often means their condition deteriorates even further.
Nor do mental health crises happen during office hours. I struggled the most late at night, long after everyone else had gone to bed. I needed support during those times when I was most liable to hurt myself, not in the mornings and afternoons when I was at work.
In this sense, a 24/7 chatbot makes lots of sense. “I don't think we should stifle innovation in this space,” Schueller says. “Because if there was any system that needs to be innovated, it's mental health services, because they are sadly insufficient. They’re terrible.”
But before building a chatbot and releasing it, Tina Hernandez-Boussard, a data scientist at Stanford Medicine, says that developers need to pause and consult with the communities they hope to serve. It requires a deep understanding of what their needs are, the language they use to describe their concerns, existing resources, and what kinds of topics and suggestions aren’t helpful. Even asking a simple question at the beginning of a conversation such as “Do you want to talk to an AI or a human?” could allow those individuals to pick the type of interaction that suits their needs, Hernandez-Boussard says.
NEDA did none of these things before deploying Tessa. The researchers who developed the online body positivity self-help program upon which Tessa was initially based created a set of online question-and-answer exercises to improve body image. It didn’t involve generative AI that could write its own answers. The bot deployed by NEDA did use generative AI, something that no one in the eating disorder community was aware of before Tessa was brought online. Consulting those with lived experience would have flagged Tessa’s weight loss and “healthy eating” recommendations, Conason says.
The question for healthcare isn’t whether to use AI, but how.
NEDA did not comment on initial Tessa’s development and deployment, but a spokesperson told Leaps.org that “Tessa will be back online once we are confident that the program will be run with the rule-based approach as it was designed.”
The tech and therapist collaboration
The question for healthcare isn’t whether to use AI, but how. Already, AI can spot anomalies on medical images with greater precision than human eyes and can flag specific areas of an image for a radiologist to review in greater detail. Similarly, in mental health, AI should be an add-on for therapy, not a counselor-in-a-box, says Aniket Bera, an expert on AI and mental health at Purdue University.
“If [AIs] are going to be good helpers, then we need to understand humans better,” Bera says. That means understanding what patients and therapists alike need help with and respond to.
One of the biggest challenges of struggling with chronic illness is the dehumanization that happens. You become a patient number, a set of laboratory values and test scores. Treatment is often dictated by invisible algorithms and rules that you have no control over or access to. It’s frightening and maddening. But this doesn’t mean chatbots don’t have any place in medicine and mental health. An AI system could help provide appointment reminders and answer procedural questions about parking and whether someone should fast before a test or a procedure. They can help manage billing and even provide support between outpatient sessions by offering suggestions for what coping skills to use, the best ways to manage anxiety, and point to local resources. As the bots get better, they may eventually shoulder more and more of the burden of providing mental health care. But as Maxwell learned with Tessa, it’s still no replacement for human interaction.
“I'm not suggesting we should go in and start replacing therapists with technologies,” Schueller says. Instead, he advocates for a therapist-tech collaboration. “The technology side and the human component—these things need to come together.”