Researchers Are Testing a New Stem Cell Therapy in the Hopes of Saving Millions from Blindness

NIH researchers in Kapil Bharti's lab work toward the development of induced pluripotent stem cells to treat dry age-related macular degeneration.
Of all the infirmities of old age, failing sight is among the cruelest. It can mean the end not only of independence, but of a whole spectrum of joys—from gazing at a sunset or a grandchild's face to reading a novel or watching TV.
The Phase 1 trial will likely run through 2022, followed by a larger Phase 2 trial that could last another two or three years.
The leading cause of vision loss in people over 55 is age-related macular degeneration, or AMD, which afflicts an estimated 11 million Americans. As photoreceptors in the macula (the central part of the retina) die off, patients experience increasingly severe blurring, dimming, distortions, and blank spots in one or both eyes.
The disorder comes in two varieties, "wet" and "dry," both driven by a complex interaction of genetic, environmental, and lifestyle factors. It begins when deposits of cellular debris accumulate beneath the retinal pigment epithelium (RPE)—a layer of cells that nourish and remove waste products from the photoreceptors above them. In wet AMD, this process triggers the growth of abnormal, leaky blood vessels that damage the photoreceptors. In dry AMD, which accounts for 80 to 90 percent of cases, RPE cells atrophy, causing photoreceptors to wither away. Wet AMD can be controlled in about a quarter of patients, usually by injections of medication into the eye. For dry AMD, no effective remedy exists.
Stem Cells: Promise and Perils
Over the past decade, stem cell therapy has been widely touted as a potential treatment for AMD. The idea is to augment a patient's ailing RPE cells with healthy ones grown in the lab. A few small clinical trials have shown promising results. In a study published in 2018, for example, a University of Southern California team cultivated RPE tissue from embryonic stem cells on a plastic matrix and transplanted it into the retinas of four patients with advanced dry AMD. Because the trial was designed to test safety rather than efficacy, lead researcher Amir Kashani told a reporter, "we didn't expect that replacing RPE cells would return a significant amount of vision." Yet acuity improved substantially in one recipient, and the others regained their lost ability to focus on an object.
Therapies based on embryonic stem cells, however, have two serious drawbacks: Using fetal cell lines raises ethical issues, and such treatments require the patient to take immunosuppressant drugs (which can cause health problems of their own) to prevent rejection. That's why some experts favor a different approach—one based on induced pluripotent stem cells (iPSCs). Such cells, first produced in 2006, are made by returning adult cells to an undifferentiated state, and then using chemicals to reprogram them as desired. Treatments grown from a patient's own tissues could sidestep both hurdles associated with embryonic cells.
At least hypothetically. Today, the only stem cell therapies approved by the U.S. Food and Drug Administration (FDA) are umbilical cord-derived products for various blood and immune disorders. Although scientists are probing the use of embryonic stem cells or iPSCs for conditions ranging from diabetes to Parkinson's disease, such applications remain experimental—or fraudulent, as a growing number of patients treated at unlicensed "stem cell clinics" have painfully learned. (Some have gone blind after receiving bogus AMD therapies at those facilities.)
Last December, researchers at the National Eye Institute in Bethesda, Maryland, began enrolling patients with dry AMD in the country's first clinical trial using tissue grown from the patients' own stem cells. Led by biologist Kapil Bharti, the team intends to implant custom-made RPE cells in 12 recipients. If the effort pans out, it could someday save the sight of countless oldsters.
That, however, is what's technically referred to as a very big "if."
The First Steps
Bharti's trial is not the first in the world to use patient-derived iPSCs to treat age-related macular degeneration. In 2013, Japanese researchers implanted such cells into the eyes of a 77-year-old woman with wet AMD; after a year, her vision had stabilized, and she no longer needed injections to keep abnormal blood vessels from forming. A second patient was scheduled for surgery—but the procedure was canceled after the lab-grown RPE cells showed signs of worrisome mutations. That incident illustrates one potential problem with using stem cells: Under some circumstances, the cells or the tissue they form could turn cancerous.
"The knowledge and expertise we're gaining can be applied to many other iPSC-based therapies."
Bharti and his colleagues have gone to great lengths to avoid such outcomes. "Our process is significantly different," he told me in a phone interview. His team begins with patients' blood stem cells, which appear to be more genomically stable than the skin cells that the Japanese group used. After converting the blood cells to RPE stem cells, his team cultures them in a single layer on a biodegradable scaffold, which helps them grow in an orderly manner. "We think this material gives us a big advantage," Bharti says. The team uses a machine-learning algorithm to identify optimal cell structure and ensure quality control.
It takes about six months for a patch of iPSCs to become viable RPE cells. When they're ready, a surgeon uses a specially-designed tool to insert the tiny structure into the retina. Within days, the scaffold melts away, enabling the transplanted RPE cells to integrate fully into their new environment. Bharti's team initially tested their method on rats and pigs with eye damage mimicking AMD. The study, published in January 2019 in Science Translational Medicine, found that at ten weeks, the implanted RPE cells continued to function normally and protected neighboring photoreceptors from further deterioration. No trace of mutagenesis appeared.
Encouraged by these results, Bharti began recruiting human subjects. The Phase 1 trial will likely run through 2022, followed by a larger Phase 2 trial that could last another two or three years. FDA approval would require an even larger Phase 3 trial, with a decision expected sometime between 2025 and 2028—that is, if nothing untoward happens before then. One unknown (among many) is whether implanted cells can thrive indefinitely under the biochemically hostile conditions of an eye with AMD.
"Most people don't have a sense of just how long it takes to get something like this to work, and how many failures—even disasters—there are along the way," says Marco Zarbin, professor and chair of Ophthalmology and visual science at Rutgers New Jersey Medical School and co-editor of the book Cell-Based Therapy for Degenerative Retinal Diseases. "The first kidney transplant was done in 1933. But the first successful kidney transplant was in 1954. That gives you a sense of the time frame. We're really taking the very first steps in this direction."
Looking Ahead
Even if Bharti's method proves safe and effective, there's the question of its practicality. "My sense is that using induced pluripotent stem cells to treat the patient from whom they're derived is a very expensive undertaking," Zarbin observes. "So you'd have to have a very dramatic clinical benefit to justify that cost."
Bharti concedes that the price of iPSC therapy is likely to be high, given that each "dose" is formulated for a single individual, requires months to manufacture, and must be administered via microsurgery. Still, he expects economies of scale and production to emerge with time. "We're working on automating several steps of the process," he explains. "When that kicks in, a technician will be able to make products for 10 or 20 people at once, so the cost will drop proportionately."
Meanwhile, other researchers are pressing ahead with therapies for AMD using embryonic stem cells, which could be mass-produced to treat any patient who needs them. But should that approach eventually win FDA approval, Bharti believes there will still be room for a technique that requires neither fetal cell lines nor immunosuppression.
And not only for eye ailments. "The knowledge and expertise we're gaining can be applied to many other iPSC-based therapies," says the scientist, who is currently consulting with several companies that are developing such treatments. "I'm hopeful that we can leverage these approaches for a wide range of applications, whether it's for vision or across the body."
NEI launches iPS cell therapy trial for dry AMD
Will the Pandemic Propel STEM Experts to Political Power?
The White House rising out of a Petri dish.
If your car won't run, you head to a mechanic. If your faucet leaks, you contact a plumber. But what do you do if your politics are broken? You call a… lawyer.
"Scientists have been more engaged with politics over the past three years amid a consistent sidelining of science and expertise, and now the pandemic has crystalized things even more."
That's been the American way since the beginning. Thousands of members of the House and Senate have been attorneys, along with nearly two dozen U.S. presidents from John Adams to Abraham Lincoln to Barack Obama. But a band of STEM professionals is changing the equation. They're hoping anger over the coronavirus pandemic will turn their expertise into a political superpower that propels more of them into office.
"This could be a turning point, part of an acceleration of something that's already happening," said Nancy Goroff, a New York chemistry professor who's running for a House seat in Long Island and will apparently be the first female scientist with a Ph.D. in Congress. "Scientists have been more engaged with politics over the past three years amid a consistent sidelining of science and expertise, and now the pandemic has crystalized things even more."
Professionals in the science, technology, engineering and medicine (STEM) fields don't have an easy task, however. To succeed, they must find ways to engage with voters instead of their usual target audiences — colleagues, patients and students. And they'll need to beat back a long-standing political tradition that has made federal and state politics a domain of attorneys and businesspeople, not nurses and biologists.
In the 2017-2018 Congress, more members of Congress said they'd worked as radio talk show hosts (seven) and as car dealership owners (six) than scientists (three — a physicist, a microbiologist, and a chemist), according to a 2018 report from the Congressional Research Service. There were more bankers (18) than physicians (14), more management consultants (18) than engineers (11), and more former judges (15) than dentists (4), nurses (2), veterinarians (3), pharmacists (1) and psychologists (3) combined.
In 2018, a "STEM wave" brought nine members with STEM backgrounds into office. But those with initials like PhD, MD and RN after their names are still far outnumbered by Esq. and MBA types.
Why the gap? Astrophysicist Rush Holt Jr., who served from 1999-2015 as a House representative from New Jersey, thinks he knows. "I have this very strong belief, based on 16 years in Congress and a long, intense public life, that the problem is not with science or the scientists," said. "It has to do with the fact that the public just doesn't pay attention to science. It never occurs to them that they have any role in the matter."
But Holt, former chief executive of the American Association for the Advancement of Science, believes change is on the way. "It's likely that the pandemic will affect people's attitudes," former congressman Holt said, "and lead them to think that they need more scientific thinking in policy-making and legislating." Holt's father was a U.S. senator from West Virginia, so he grew up with a political education. But how can scientists and medical professionals succeed if they have no background in the art of wooing voters?
That's where an organization called 314 Action comes in. Named after the first three digits of pi, 314 Action declares itself to be the "pro-science resistance" and says it's trained more than 1,400 scientists to run for public office.
In 2018, 9 out of 13 House and Senate candidates endorsed by the group won their races. In 2020, 314 Action is endorsing 12 candidates for the House (including an engineer), four for the Senate (including an astronaut) and one for governor (a mathematician in Kansas). It expects to spend $10 million-$20 million to support campaigns this year.
"Physicians, scientists and engineers are problem-solvers," said Shaughnessy Naughton, a Pennsylvania chemist who founded 314 Action after an unsuccessful bid for Congress. "They're willing to dive into issues, and their skills would benefit policy decisions that extend way beyond their scientific fields of expertise."
Like many political organizations, 314 Action focuses on teaching potential candidate how to make it in politics, aiming to help them drop habits that fail to bridge the gap between scientists and civilians. "Their first impulse is not to tell a story," public speaking coach Chris Jahnke told the public radio show "Marketplace" in 2018. "They would rather start with a stat." In a training session, Jahnke aimed to teach them to do both effectively.
"It just comes down to being able to speak about general principles in regular English, and to always have the science intertwined with basic human values," said Rep. Kim Schrier, a Washington state pediatrician who won election to Congress in 2018.
She believes her experience on the job has helped her make connections with voters. In a chat with parents about vaccines for their child, for example, she knows not to directly jump into an arcane discussion of case-control studies.
The best alternative, she said, is to "talk about how hard it is to be a parent making these decisions, feeling scared and worried. Then say that you've looked at the data and the research, and point out that pediatricians would never do anything to hurt children because we want to do everything that is good for them. When you speak heart to heart, it gets across the message and the credibility of medicine and science."
The pandemic "will hopefully awaken people and trigger a change that puts science, medicine and public health on a pedestal where science is revered and not dismissed as elitist."
Communication skills will be especially important if the pandemic spurs more Americans to focus on politics and the records of incumbents in regard to matters like public health and climate change. Thousands of candidates will have to address the nation's coronavirus response, and a survey commissioned by 314 Action suggests that voters may be receptive to those with STEM backgrounds. The poll, of 1,002 likely voters in early April 2020, found that 41%-46% of those surveyed said they'd be "much more favorable" toward candidates who were doctors, nurses, scientists and public health professionals. Those numbers were the highest in the survey compared to just 9% for lawyers.
The pandemic "will hopefully awaken people and trigger a change that puts science, medicine and public health on a pedestal where science is revered and not dismissed as elitist," Dr. Schrier said. "It will come from a recognition that what's going to get us out of this bind are scientists, vaccine development and the hard work of the people in public health on the ground."
[This article was originally published on June 8th, 2020 as part of a standalone magazine called GOOD10: The Pandemic Issue. Produced as a partnership among LeapsMag, The Aspen Institute, and GOOD, the magazine is available for free online.]
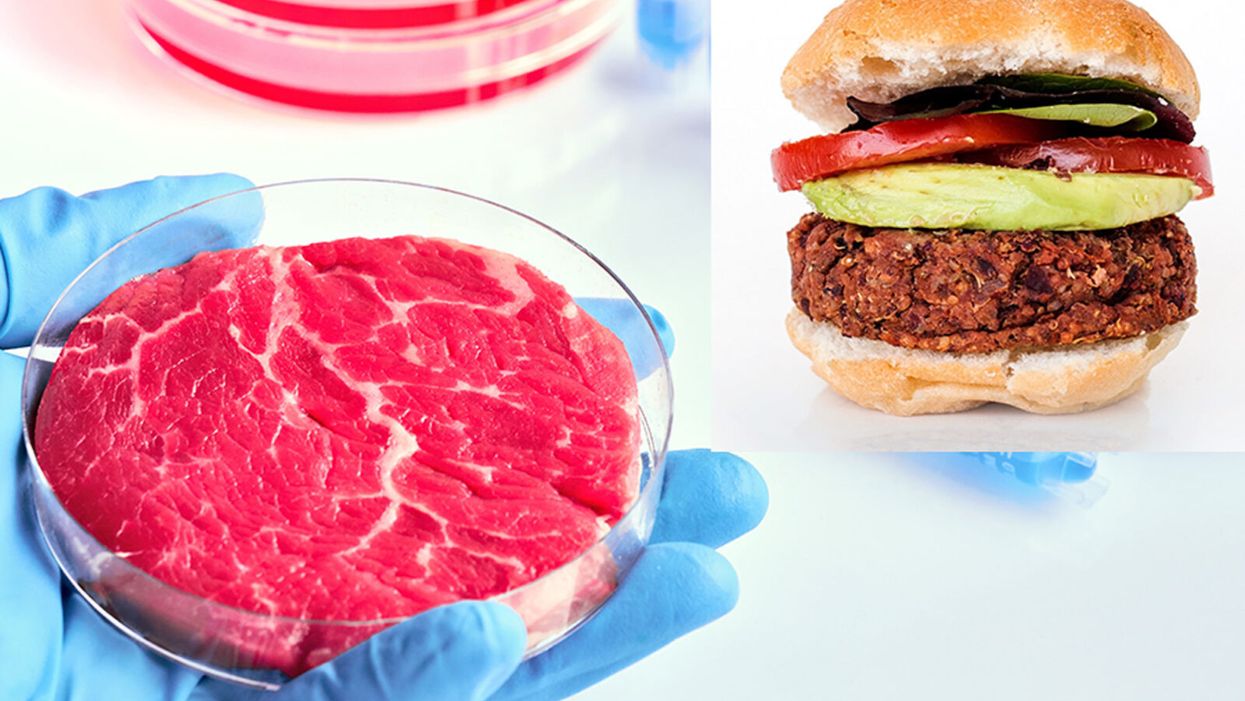
While lab-grown meat is not yet ready to crisis-proof the food supply chain, it offers key benefits that could make it an important solution beyond this pandemic.
The coronavirus pandemic exposed significant weaknesses in the country's food supply chain. Grocery store meat counters were bare. Transportation interruptions influenced supply. Finding beef, poultry, and pork at the store has been, in some places, as challenging as finding toilet paper.
In traditional agriculture models, it takes at least three months to raise chicken, six to nine months for pigs, and 18 months for cattle.
It wasn't a lack of supply -- millions of animals were in the pipeline.
"There's certainly enough food out there, but it can't get anywhere because of the way our system is set up," said Amy Rowat, an associate professor of integrative biology and physiology at UCLA. "Having a more self-contained, self-sufficient way to produce meat could make the supply chain more robust."
Cultured meat could be one way of making the meat supply chain more resilient despite disruptions due to pandemics such as COVID-19. But is the country ready to embrace lab-grown food?
According to a Good Food Institute study, GenZ is almost twice as likely to embrace meat alternatives for reasons related to social and environmental awareness, even prior to the pandemic. That's because this group wants food choices that reflect their values around food justice, equity, and animal welfare.
Largely, the interest in protein alternatives has been plant-based foods. However, factors directly related to COVID-19 may accelerate consumer interest in the scaling up of cell-grown products, according to Liz Specht, the associate director of science and technology at The Good Food Institute. The latter is a nonprofit organization that supports scientists, investors, and entrepreneurs working to develop food alternatives to conventional animal products.
While lab-grown food isn't ready yet to definitively crisis-proof the food supply chain, experts say it offers promise.
Matching Supply and Demand
Companies developing cell-grown meat claim it can take as few as two months to develop a cell into an edible product, according to Anthony Chow, CFA at Agronomics Limited, an investment company focused on meat alternatives. Tissue is taken from an animal and placed in a culture that contains nutrients and proteins the cells need to grow and expand. He cites a Good Food Institute report that claims a 2.5-millimeter sample can grow three and a half tons of meat in 40 days, allowing for exponential growth when needed.
In traditional agriculture models, it takes at least three months to raise chicken, six to nine months for pigs, and 18 months for cattle. To keep enough maturing animals in the pipeline, farms must plan the number of animals to raise months -- even years -- in advance. Lab-grown meat advocates say that because cultured meat supplies can be flexible, it theoretically allows for scaling up or down in significantly less time.
"Supply and demand has drastically changed in some way around the world and cultivated meat processing would be able to adapt much quicker than conventional farming," Chow said.
Scaling Up
Lab-grown meat may provide an eventual solution, but not in the immediate future, said Paul Mozdziak, a professor of physiology at North Carolina State University who researches animal cell culture techniques, transgenic animal production, and muscle biology.
"The challenge is in culture media," he said. "It's going to take some innovation to get the cells to grow at quantities that are going to be similar to what you can get from an animal. These are questions that everybody in the space is working on."
Chow says some of the most advanced cultured meat companies, such as BlueNal, anticipate introducing products to the market midway through next year. However, he thinks COVID-19 has slowed the process. Once introduced, they will be at a premium price, most likely available at restaurants before they hit grocery store shelves.
"I think in five years' time it will be in a different place," he said. "I don't think that this will have relevance for this pandemic, but certainly beyond that."
"Plant-based meats may be perceived as 'alternatives' to meat, whereas lab-grown meat is producing the same meat, just in a much more efficient manner, without the environmental implications."
Of course, all the technological solutions in the world won't solve the problem unless people are open-minded about embracing them. At least for now, a lab-grown burger or bluefin tuna might still be too strange for many people, especially in the U.S.
For instance, a 2019 article published by "Frontiers in Sustainable Food Systems" reflects results from a study of 3,030 consumers showing that 29 percent of U.S. customers, 59 percent of Chinese consumers, and 56 percent of Indian consumers were either 'very' or 'extremely likely' to try cultivated meat.
"Lab-grown meat is genuine meat, at the cellular level, and therefore will match conventional meat with regard to its nutritional content and overall sensory experience. It could be argued that plant-based meat will never be able to achieve this," says Laura Turner, who works with Chow at Agronomics Limited. "Plant-based meats may be perceived as 'alternatives' to meat, whereas lab-grown meat is producing the same meat, just in a much more efficient manner, without the environmental implications."
A Solution Beyond This Pandemic
The coronavirus has done more than raise awareness of the fragility of food supply chains. It has also been a wakeup call for consumers and policy makers that it is time to radically rethink our meat, Specht says. Those factors have elevated the profile of lab-grown meat.
"I think the economy is getting a little bit more steam and if I was an investor, I would be getting excited about it," adds Mozdziak.
Beyond crises, Mozdziak explains that as affluence continues to increase globally, meat consumption increases exponentially. Yet farm animals can only grow so quickly and traditional farming won't be able to keep up.
"Even Tyson is saying that by 2050, there's not going to be enough capacity in the animal meat space to meet demand," he notes. "If we don't look at some innovative technologies, how are we going to overcome that?"