Researchers Are Testing a New Stem Cell Therapy in the Hopes of Saving Millions from Blindness

NIH researchers in Kapil Bharti's lab work toward the development of induced pluripotent stem cells to treat dry age-related macular degeneration.
Of all the infirmities of old age, failing sight is among the cruelest. It can mean the end not only of independence, but of a whole spectrum of joys—from gazing at a sunset or a grandchild's face to reading a novel or watching TV.
The Phase 1 trial will likely run through 2022, followed by a larger Phase 2 trial that could last another two or three years.
The leading cause of vision loss in people over 55 is age-related macular degeneration, or AMD, which afflicts an estimated 11 million Americans. As photoreceptors in the macula (the central part of the retina) die off, patients experience increasingly severe blurring, dimming, distortions, and blank spots in one or both eyes.
The disorder comes in two varieties, "wet" and "dry," both driven by a complex interaction of genetic, environmental, and lifestyle factors. It begins when deposits of cellular debris accumulate beneath the retinal pigment epithelium (RPE)—a layer of cells that nourish and remove waste products from the photoreceptors above them. In wet AMD, this process triggers the growth of abnormal, leaky blood vessels that damage the photoreceptors. In dry AMD, which accounts for 80 to 90 percent of cases, RPE cells atrophy, causing photoreceptors to wither away. Wet AMD can be controlled in about a quarter of patients, usually by injections of medication into the eye. For dry AMD, no effective remedy exists.
Stem Cells: Promise and Perils
Over the past decade, stem cell therapy has been widely touted as a potential treatment for AMD. The idea is to augment a patient's ailing RPE cells with healthy ones grown in the lab. A few small clinical trials have shown promising results. In a study published in 2018, for example, a University of Southern California team cultivated RPE tissue from embryonic stem cells on a plastic matrix and transplanted it into the retinas of four patients with advanced dry AMD. Because the trial was designed to test safety rather than efficacy, lead researcher Amir Kashani told a reporter, "we didn't expect that replacing RPE cells would return a significant amount of vision." Yet acuity improved substantially in one recipient, and the others regained their lost ability to focus on an object.
Therapies based on embryonic stem cells, however, have two serious drawbacks: Using fetal cell lines raises ethical issues, and such treatments require the patient to take immunosuppressant drugs (which can cause health problems of their own) to prevent rejection. That's why some experts favor a different approach—one based on induced pluripotent stem cells (iPSCs). Such cells, first produced in 2006, are made by returning adult cells to an undifferentiated state, and then using chemicals to reprogram them as desired. Treatments grown from a patient's own tissues could sidestep both hurdles associated with embryonic cells.
At least hypothetically. Today, the only stem cell therapies approved by the U.S. Food and Drug Administration (FDA) are umbilical cord-derived products for various blood and immune disorders. Although scientists are probing the use of embryonic stem cells or iPSCs for conditions ranging from diabetes to Parkinson's disease, such applications remain experimental—or fraudulent, as a growing number of patients treated at unlicensed "stem cell clinics" have painfully learned. (Some have gone blind after receiving bogus AMD therapies at those facilities.)
Last December, researchers at the National Eye Institute in Bethesda, Maryland, began enrolling patients with dry AMD in the country's first clinical trial using tissue grown from the patients' own stem cells. Led by biologist Kapil Bharti, the team intends to implant custom-made RPE cells in 12 recipients. If the effort pans out, it could someday save the sight of countless oldsters.
That, however, is what's technically referred to as a very big "if."
The First Steps
Bharti's trial is not the first in the world to use patient-derived iPSCs to treat age-related macular degeneration. In 2013, Japanese researchers implanted such cells into the eyes of a 77-year-old woman with wet AMD; after a year, her vision had stabilized, and she no longer needed injections to keep abnormal blood vessels from forming. A second patient was scheduled for surgery—but the procedure was canceled after the lab-grown RPE cells showed signs of worrisome mutations. That incident illustrates one potential problem with using stem cells: Under some circumstances, the cells or the tissue they form could turn cancerous.
"The knowledge and expertise we're gaining can be applied to many other iPSC-based therapies."
Bharti and his colleagues have gone to great lengths to avoid such outcomes. "Our process is significantly different," he told me in a phone interview. His team begins with patients' blood stem cells, which appear to be more genomically stable than the skin cells that the Japanese group used. After converting the blood cells to RPE stem cells, his team cultures them in a single layer on a biodegradable scaffold, which helps them grow in an orderly manner. "We think this material gives us a big advantage," Bharti says. The team uses a machine-learning algorithm to identify optimal cell structure and ensure quality control.
It takes about six months for a patch of iPSCs to become viable RPE cells. When they're ready, a surgeon uses a specially-designed tool to insert the tiny structure into the retina. Within days, the scaffold melts away, enabling the transplanted RPE cells to integrate fully into their new environment. Bharti's team initially tested their method on rats and pigs with eye damage mimicking AMD. The study, published in January 2019 in Science Translational Medicine, found that at ten weeks, the implanted RPE cells continued to function normally and protected neighboring photoreceptors from further deterioration. No trace of mutagenesis appeared.
Encouraged by these results, Bharti began recruiting human subjects. The Phase 1 trial will likely run through 2022, followed by a larger Phase 2 trial that could last another two or three years. FDA approval would require an even larger Phase 3 trial, with a decision expected sometime between 2025 and 2028—that is, if nothing untoward happens before then. One unknown (among many) is whether implanted cells can thrive indefinitely under the biochemically hostile conditions of an eye with AMD.
"Most people don't have a sense of just how long it takes to get something like this to work, and how many failures—even disasters—there are along the way," says Marco Zarbin, professor and chair of Ophthalmology and visual science at Rutgers New Jersey Medical School and co-editor of the book Cell-Based Therapy for Degenerative Retinal Diseases. "The first kidney transplant was done in 1933. But the first successful kidney transplant was in 1954. That gives you a sense of the time frame. We're really taking the very first steps in this direction."
Looking Ahead
Even if Bharti's method proves safe and effective, there's the question of its practicality. "My sense is that using induced pluripotent stem cells to treat the patient from whom they're derived is a very expensive undertaking," Zarbin observes. "So you'd have to have a very dramatic clinical benefit to justify that cost."
Bharti concedes that the price of iPSC therapy is likely to be high, given that each "dose" is formulated for a single individual, requires months to manufacture, and must be administered via microsurgery. Still, he expects economies of scale and production to emerge with time. "We're working on automating several steps of the process," he explains. "When that kicks in, a technician will be able to make products for 10 or 20 people at once, so the cost will drop proportionately."
Meanwhile, other researchers are pressing ahead with therapies for AMD using embryonic stem cells, which could be mass-produced to treat any patient who needs them. But should that approach eventually win FDA approval, Bharti believes there will still be room for a technique that requires neither fetal cell lines nor immunosuppression.
And not only for eye ailments. "The knowledge and expertise we're gaining can be applied to many other iPSC-based therapies," says the scientist, who is currently consulting with several companies that are developing such treatments. "I'm hopeful that we can leverage these approaches for a wide range of applications, whether it's for vision or across the body."
NEI launches iPS cell therapy trial for dry AMD
Scientists Just Created Liquid Solar Power That Can Be Stored for 18 Years
A team of Swedish scientists has gathered solar power so pure, that until recently, capturing it was an impossibility.
Look no further than this week's climate strikes for evidence that millions of people are passionate about curbing global warming.
Unlike relatively limited solar panel energy storage, norbornadiene can potentially maintain its potency for years.
But even potential solutions, like alternative meats, have their own challenges. Some scientists are putting their focus on the sun to help balance out our energy consumption.
In fact, they are gathering solar power so pure that, until recently, capturing it was an impossibility.
The Lowdown
A group of Swedish scientists has created a liquid called norbornadiene. This liquid sunshine can capture up to 30 percent of raw solar power. To put it in perspective, the best publicly available solar panels can harness 21 percent. Norbornadiene would bring in about 50 percent more power – a significant difference in energy efficiency.
Most notably, unlike relatively limited solar panel energy storage, norbornadiene can potentially maintain its potency for years. We could have the ability to collect and store premium solar power, making it easier for current and future generations to use fossil and nuclear fuel alternatives.
"The norbornadiene molecules that we have made have very good properties, in terms of solar energy capture efficiency, storage time and energy density," says team lead Dr. Kasper Moth-Poulson of the Chamlers University of Technology. "They can store energy without the need for insulation materials for 18 or more years."
Next Up
Swedish scientist Moth-Poulsen and his team have been testing the norbornadiene on the physics building roof at the Chalmers University of Technology. Once activated, it heats up to just below boiling and provides enough power to be useful.
The energy density is 250 watt-hours per kilogram, twice the strength of Tesla's popular Powerall battery.
It requires potentially toxic solvents, like a cobalt-based activator, to transform into its full potential. The team is currently trying to find less-hazardous catalysts to help transform the norbornadiene to its active form, quadricyclane. Exposing it to sunlight is the main way to reactivate the norbornadiene's power. Over time, scientists will likely make it more efficient with less toxic agents.
The energy density is 250 watt-hours per kilogram, twice the strength of Tesla's popular Powerall battery.
Open Questions
The biggest question is safety, perceived or otherwise: Are you ready to drive around with 250 kWh of pure solar in your Hyundai? Norbornadiene may be stable in a hermetically sealed lab, but sculpting it for everyday use requires another level of security.
The half-life of the sunshine power is also an estimate, too. The challenge with new scientific substances is you don't know how the matter will evolve over time. It is easy to be overly optimistic about this one discovery being the key to our energy needs. For the time being, it is wiser to look at norbornadiene as a progressive step rather than a revolutionary one.
Even at its least effective, norbornadiene and its related material is a step toward us utilizing the one natural resource that won't run out for generations. In the short-term, a stable form of it could offset our fossil and nuclear fuel use and even help lower the carbon footprint made by long-distance transportation. It will be fascinating to see what future aircraft builders, home designers and even car manufacturers do as the solar technology conversation heats up.
Moth-Poulsen wants norbornadiene to be a definitive part of the climate change puzzle.
"I hope that in five years, we will see the first products based on our molecules and could help mitigate the daily variations in temperature," he says. "This will lead to increased thermal comfort and reduced energy consumption for heating and cooling."
Virtual Reality is Making Medical Care for Kids Less Scary and Painful
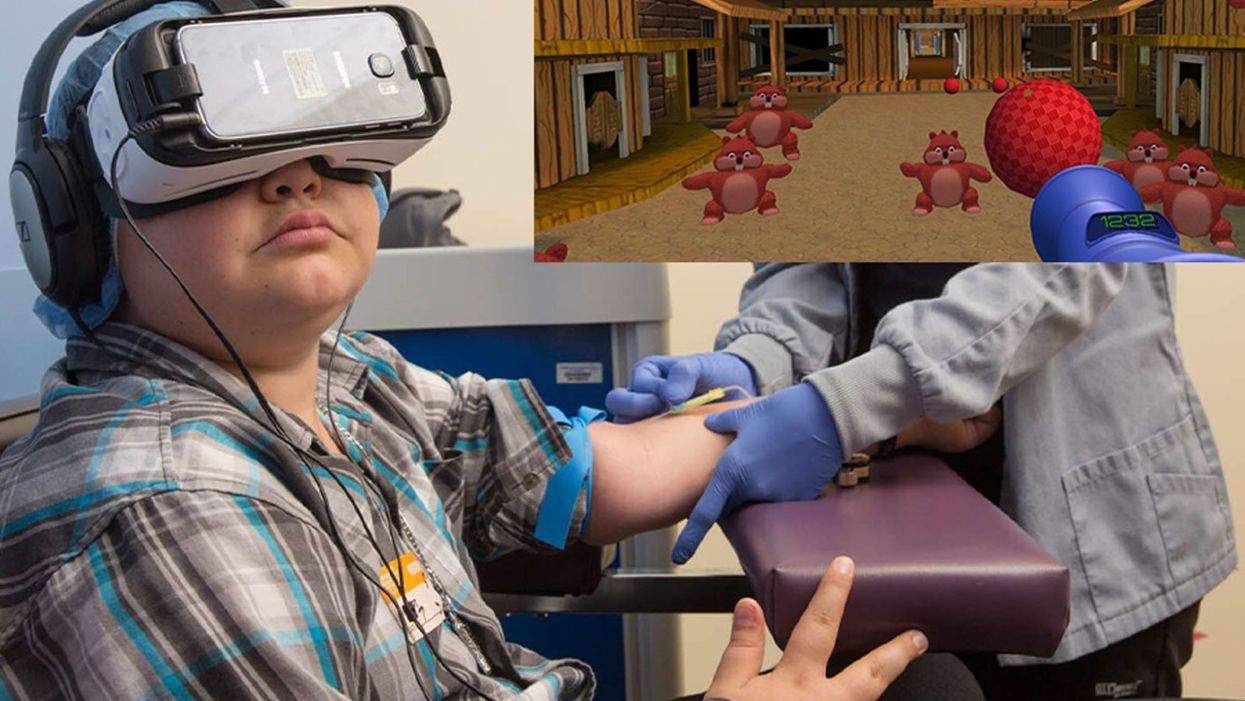
A patient at Children's Hospital Los Angeles (Courtesy of Children's Hospital Los Angeles) plus Bear Blast, developed by AppliedVR.
A blood draw is not normally a fun experience, but these days, virtual reality technology is changing that.
Instead of watching a needle go into his arm, a child wearing a VR headset at Children's Hospital Los Angeles can play a game throwing balls at cartoon bears. In Seattle, at the University of Washington, a burn patient can immerse herself in a soothing snow scene. And at the University of Miami Hospital, a five-minute skin biopsy can become an exciting ride at an amusement park.
VR is transforming once-frightening medical encounters for kids, from blood draws to biopsies to pre-surgical prep, into tolerable ones.
It's literally a game changer, says pediatric neurosurgeon Kurtis Auguste, who uses the tool to help explain pending operations to his young patients and their families. The virtual reality 3-D portrait of their brain is recreated from an MRI, originally to help plan the surgery. The image of normally bland tissue is painted with false colors to better see the boundaries and anomalies of each component. It can be rotated, viewed from every possible angle, zoomed in and out; incisions can be made and likely results anticipated. Auguste has extended its use to patients and families.
"The moment you put these headsets on the kids, we immediately have a link, because honestly, this is how they communicate with each other," says Auguste. "We're all sitting around the table playing games. It's really bridged the distance between me, the pediatric specialist, and my patients" at the Benioff Children's Hospital Oakland, now affiliated with the University of California San Francisco School of Medicine.
The VR experience engages people where they are, immersing them in the environment rather than lecturing them. And it seems to work in all environments, across age and cultural differences, leading to a better grasp of what will be undertaken. That understanding is crucial to meaningful informed consent for surgery. It is particularly relevant for safety-net hospitals, which includes most children's hospitals, because often members of the families were born elsewhere and may have limited understanding of English, not to mention advanced medicine.
Targeting pain
"We're trying to target ways that we can decrease pain, anxiety, fear – what people usually experience as a function of a needle," says Jeffrey Gold, a pioneer in adapting VR at Children's Hospital Los Angeles. He ran the pain clinic there and in 2004 initially focused on phlebotomy, simple blood draws. Many of their kids require frequent blood draws to monitor serious chronic conditions such as diabetes, HIV infection, sickle cell disease, and other conditions that affect the heart, liver, kidneys and other organs.
The scientific explanation of how VR works for pain relief draws upon two basic principles of brain function. The first is "top down inhibition," Gold explains. "We all have the inherent capacity to turn down signals once we determine that signal is no longer harmful, dangerous, hurtful, etc. That's how our brain operates on purpose. It's not just a distraction, it's actually your brain stopping the pain signal at the spinal cord before it can fire all the way up to the frontal lobe."
Second is the analgesic effect from endorphins. "If you're in a gaming environment, and you're having fun and you're laughing and giggling, you are actually releasing endorphins...a neurochemical reaction at the synaptic level of the brain," he says.
Part of what makes VR effective is "what's called a cognitive load, where you have to actually learn something and do something," says Gold. He has worked with developers on a game call Bear Blast, which has proven to be effective in a clinical trial for mitigating pain. But he emphasizes, it is not a one-size-fits all; the programs and patients need to be evaluated to understand what works best for each case.
Gold was a bit surprised to find that VR "actually facilitates quicker blood draws," because the staff doesn't have to manage the kids' anxiety, so "they require fewer needle sticks." The kids, parents, and staff were all having a good time, "and that's a big win when everybody is benefiting." About two years ago the hospital made VR an option that patients can request in the phlebotomy lab, and about half of kids age 4 and older choose to do so.
The technology "gets the kids engaged and performing the activity the way we want them to" to maximize recovery.
VR reduces or eliminates the need to use sedation or anesthesia, which carries a small but real risk of an adverse reaction. And important to parents, it eliminates the recovery time from using sedation, which shortens the visit and time missed from school and work.
A more intriguing question is whether reducing fear and anxiety in early-life experiences with the healthcare system through activities like VR will have a long-term affect on kids' attitudes toward medicine as they grow older. "If you're a screaming meemie when you come get your blood draw when you're five or seven, you're still that anxious adolescent or adult who is all quivering and sweating and avoiding healthcare," Gold says. "That's a longitudinal health outcome I'd love to get my hands on in 10-15 years from now."
Broader applications
Dermatologist Hadar Lev-Tov read about the use of VR to treat pain and decided to try it in his practice at the University of Miami Hospital. He thought, "OK, this is low risk, it's easy to do. So we got some equipment and got it done." It was so affordable he paid for it out of his own pocket, rather than wait to go through administrative channels. The results were so interesting that he decided to publish it as a series of case studies with a wide variety of patients and types of procedures.
Some of them, such as freezing off warts, are not particularly painful. "But there can be a lot of anxiety, especially for kids, which can be worse than pain and can disrupt the procedure." It can trigger a non-rational, primal fight or flight response in the limbic region of the brain.
Adults understand the need for a biopsy of a skin growth and tolerate what might be a momentary flick of pain. "But for a kid you think twice about a biopsy, both because it's a hassle and because it could be a traumatic event for a child," says Lev-Tov. VR has helped to allay such fears and improve medical care.
Integrating VR into practice has been relatively easy, primarily focusing on simple training for staff and ensuring that standard infection control practices are used in handling equipment that is used by different patients. More mundane issues are ensuring that the play back and wi-fi equipment are functioning properly. He has had a few complaints from kids when the procedure is competed and the VR is turned off prematurely, which is why he favors programs like a roller coaster ride that lasts about five minutes, ample time to take a biopsy or two.
The future is today
The pediatric neurosurgeon Auguste is collaborating with colleagues at Oakland Children's to expand use of VR into different areas of care. Cancer specialists often use a port, a bubble installed under the skin in the chest of the child, to administer chemotherapy. But the young patient's curiosity often draws their attention downward to the port and their chin can potentially contaminate or obstruct it, interfering with the procedure. So the team developed a VR game involving birds that requires players to move their heads upward, away from the port, improving administration of the drugs and reducing the risk of infection.
Innovative use of VR just may be one tool that actually makes kids eager to visit the doctor.
Other games are being developed for rehabilitation that require the use of specific nerve and muscle combinations. The technology "gets the kids engaged and performing the activity the way we want them to" to maximize recovery, Auguste explains. "We can monitor their progress by the score on the game, and if it plateaus, maybe switch to another game."
Another project is trying to ease the anxiety and confusion of the patient and family experience within the hospital itself. Hospital staff are creating a personalized VR introductory walking tour that leads from the parking garage through the maze of structures and corridors in the hospital complex to Dr. Auguste's office, phlebotomy, the MRI site, and other locations they might visit. The goal is to make them familiar with key landmarks before they even set foot in the facility. "So when they come the day of the visit they have already taken that exact same path, hopefully more than once."
"They don't miss their MRI appointment and therefore they don't miss their clinical appointment with me," says Auguste. It reduces patient anxiety about the encounter and from the hospital's perspective, it will reduce costs of missed and rescheduled visits simply because patients did not go to the right place at the right time.
The VR visit will be emailed to patients ahead of time and they can watch it on a smartphone installed in a disposable cardboard viewer. Oakland Children's hopes to have the system in place by early next year. Auguste says their goal in using VR, like other health care providers across the country, is "to streamline the entire patient experience."
Innovative use of VR just may be one tool that actually makes kids eager to visit the doctor. That would be a boon to kids, parents, and the health of America.