Researchers Behaving Badly: Known Frauds Are "the Tip of the Iceberg"

Disgraced stem cell researcher and celebrity surgeon Paolo Macchiarini.
Last week, the whistleblowers in the Paolo Macchiarini affair at Sweden's Karolinska Institutet went on the record here to detail the retaliation they suffered for trying to expose a star surgeon's appalling research misconduct.
Scientific fraud of the type committed by Macchiarini is rare, but studies suggest that it's on the rise.
The whistleblowers had discovered that in six published papers, Macchiarini falsified data, lied about the condition of patients and circumvented ethical approvals. As a result, multiple patients suffered and died. But Karolinska turned a blind eye for years.
Scientific fraud of the type committed by Macchiarini is rare, but studies suggest that it's on the rise. Just this week, for example, Retraction Watch and STAT together broke the news that a Harvard Medical School cardiologist and stem cell researcher, Piero Anversa, falsified data in a whopping 31 papers, which now have to be retracted. Anversa had claimed that he could regenerate heart muscle by injecting bone marrow cells into damaged hearts, a result that no one has been able to duplicate.
A 2009 study published in the Public Library of Science (PLOS) found that about two percent of scientists admitted to committing fabrication, falsification or plagiarism in their work. That's a small number, but up to one third of scientists admit to committing "questionable research practices" that fall into a gray area between rigorous accuracy and outright fraud.
These dubious practices may include misrepresentations, research bias, and inaccurate interpretations of data. One common questionable research practice entails formulating a hypothesis after the research is done in order to claim a successful premise. Another highly questionable practice that can shape research is ghost-authoring by representatives of the pharmaceutical industry and other for-profit fields. Still another is gifting co-authorship to unqualified but powerful individuals who can advance one's career. Such practices can unfairly bolster a scientist's reputation and increase the likelihood of getting the work published.
The above percentages represent what scientists admit to doing themselves; when they evaluate the practices of their colleagues, the numbers jump dramatically. In a 2012 study published in the Journal of Research in Medical Sciences, researchers estimated that 14 percent of other scientists commit serious misconduct, while up to 72 percent engage in questionable practices. While these are only estimates, the problem is clearly not one of just a few bad apples.
In the PLOS study, Daniele Fanelli says that increasing evidence suggests the known frauds are "just the 'tip of the iceberg,' and that many cases are never discovered" because fraud is extremely hard to detect.
Essentially everyone wants to be associated with big breakthroughs, and they may overlook scientifically shaky foundations when a major advance is claimed.
In addition, it's likely that most cases of scientific misconduct go unreported because of the high price of whistleblowing. Those in the Macchiarini case showed extraordinary persistence in their multi-year campaign to stop his deadly trachea implants, while suffering serious damage to their careers. Such heroic efforts to unmask fraud are probably rare.
To make matters worse, there are numerous players in the scientific world who may be complicit in either committing misconduct or covering it up. These include not only primary researchers but co-authors, institutional executives, journal editors, and industry leaders. Essentially everyone wants to be associated with big breakthroughs, and they may overlook scientifically shaky foundations when a major advance is claimed.
Another part of the problem is that it's rare for students in science and medicine to receive an education in ethics. And studies have shown that older, more experienced and possibly jaded researchers are more likely to fudge results than their younger, more idealistic colleagues.
So, given the steep price that individuals and institutions pay for scientific misconduct, what compels them to go down that road in the first place? According to the JRMS study, individuals face intense pressures to publish and to attract grant money in order to secure teaching positions at universities. Once they have acquired positions, the pressure is on to keep the grants and publishing credits coming in order to obtain tenure, be appointed to positions on boards, and recruit flocks of graduate students to assist in research. And not to be underestimated is the human ego.
Paolo Macchiarini is an especially vivid example of a scientist seeking not only fortune, but fame. He liberally (and falsely) claimed powerful politicians and celebrities, even the Pope, as patients or admirers. He may be an extreme example, but we live in an age of celebrity scientists who bring huge amounts of grant money and high prestige to the institutions that employ them.
The media plays a significant role in both glorifying stars and unmasking frauds. In the Macchiarini scandal, the media first lifted him up, as in NBC's laudatory documentary, "A Leap of Faith," which painted him as a kind of miracle-worker, and then brought him down, as in the January 2016 documentary, "The Experiments," which chronicled the agonizing death of one of his patients.
Institutions can also play a crucial role in scientific fraud by putting more emphasis on the number and frequency of papers published than on their quality. The whole course of a scientist's career is profoundly affected by something called the h-index. This is a number based on both the frequency of papers published and how many times the papers are cited by other researchers. Raising one's ranking on the h-index becomes an overriding goal, sometimes eclipsing the kind of patient, time-consuming research that leads to true breakthroughs based on reliable results.
Universities also create a high-pressured environment that encourages scientists to cut corners. They, too, place a heavy emphasis on attracting large monetary grants and accruing fame and prestige. This can lead them, just as it led Karolinska, to protect a star scientist's sloppy or questionable research. According to Dr. Andrew Rosenberg, who is director of the Center for Science and Democracy at the U.S.-based Union of Concerned Scientists, "Karolinska defended its investment in an individual as opposed to the long-term health of the institution. People were dying, and they should have outsourced the investigation from the very beginning."
Having institutions investigate their own practices is a conflict of interest from the get-go, says Rosenberg.
Scientists, universities, and research institutions are also not immune to fads. "Hot" subjects attract grant money and confer prestige, incentivizing scientists to shift their research priorities in a direction that garners more grants. This can mean neglecting the scientist's true area of expertise and interests in favor of a subject that's more likely to attract grant money. In Macchiarini's case, he was allegedly at the forefront of the currently sexy field of regenerative medicine -- a field in which Karolinska was making a huge investment.
The relative scarcity of resources intensifies the already significant pressure on scientists. They may want to publish results rapidly, since they face many competitors for limited grant money, academic positions, students, and influence. The scarcity means that a great many researchers will fail while only a few succeed. Once again, the temptation may be to rush research and to show it in the most positive light possible, even if it means fudging or exaggerating results.
Though the pressures facing scientists are very real, the problem of misconduct is not inevitable.
Intense competition can have a perverse effect on researchers, according to a 2007 study in the journal Science of Engineering and Ethics. Not only does it place undue pressure on scientists to succeed, it frequently leads to the withholding of information from colleagues, which undermines a system in which new discoveries build on the previous work of others. Researchers may feel compelled to withhold their results because of the pressure to be the first to publish. The study's authors propose that more investment in basic research from governments could alleviate some of these competitive pressures.
Scientific journals, although they play a part in publishing flawed science, can't be expected to investigate cases of suspected fraud, says the German science blogger Leonid Schneider. Schneider's writings helped to expose the Macchiarini affair.
"They just basically wait for someone to retract problematic papers," he says.
He also notes that, while American scientists can go to the Office of Research Integrity to report misconduct, whistleblowers in Europe have no external authority to whom they can appeal to investigate cases of fraud.
"They have to go to their employer, who has a vested interest in covering up cases of misconduct," he says.
Science is increasingly international. Major studies can include collaborators from several different countries, and he suggests there should be an international body accessible to all researchers that will investigate suspected fraud.
Ultimately, says Rosenberg, the scientific system must incorporate trust. "You trust co-authors when you write a paper, and peer reviewers at journals trust that scientists at research institutions like Karolinska are acting with integrity."
Without trust, the whole system falls apart. It's the trust of the public, an elusive asset once it has been betrayed, that science depends upon for its very existence. Scientific research is overwhelmingly financed by tax dollars, and the need for the goodwill of the public is more than an abstraction.
The Macchiarini affair raises a profound question of trust and responsibility: Should multiple co-authors be held responsible for a lead author's misconduct?
Karolinska apparently believes so. When the institution at last owned up to the scandal, it vindictively found Karl Henrik-Grinnemo, one of the whistleblowers, guilty of scientific misconduct as well. It also designated two other whistleblowers as "blameworthy" for their roles as co-authors of the papers on which Macchiarini was the lead author.
As a result, the whistleblowers' reputations and employment prospects have become collateral damage. Accusations of research misconduct can be a career killer. Research grants dry up, employment opportunities evaporate, publishing becomes next to impossible, and collaborators vanish into thin air.
Grinnemo contends that co-authors should only be responsible for their discrete contributions, not for the data supplied by others.
"Different aspects of a paper are highly specialized," he says, "and that's why you have multiple authors. You cannot go through every single bit of data because you don't understand all the parts of the article."
This is especially true in multidisciplinary, translational research, where there are sometimes 20 or more authors. "You have to trust co-authors, and if you find something wrong you have to notify all co-authors. But you couldn't go through everything or it would take years to publish an article," says Grinnemo.
Though the pressures facing scientists are very real, the problem of misconduct is not inevitable. Along with increased support from governments and industry, a change in academic culture that emphasizes quality over quantity of published studies could help encourage meritorious research.
But beyond that, trust will always play a role when numerous specialists unite to achieve a common goal: the accumulation of knowledge that will promote human health, wealth, and well-being.
[Correction: An earlier version of this story mistakenly credited The New York Times with breaking the news of the Anversa retractions, rather than Retraction Watch and STAT, which jointly published the exclusive on October 14th. The piece in the Times ran on October 15th. We regret the error.]
Although Jonas Salk has gone down in history for helping rid the world (almost) of polio, his revolutionary vaccine wouldn't have been possible without the world’s largest clinical trial – and the bravery of thousands of kids.
Exactly 67 years ago, in 1955, a group of scientists and reporters gathered at the University of Michigan and waited with bated breath for Dr. Thomas Francis Jr., director of the school’s Poliomyelitis Vaccine Evaluation Center, to approach the podium. The group had gathered to hear the news that seemingly everyone in the country had been anticipating for the past two years – whether the vaccine for poliomyelitis, developed by Francis’s former student Jonas Salk, was effective in preventing the disease.
Polio, at that point, had become a household name. As the highly contagious virus swept through the United States, cities closed their schools, movie theaters, swimming pools, and even churches to stop the spread. For most, polio presented as a mild illness, and was usually completely asymptomatic – but for an unlucky few, the virus took hold of the central nervous system and caused permanent paralysis of muscles in the legs, arms, and even people’s diaphragms, rendering the person unable to walk and breathe. It wasn’t uncommon to hear reports of people – mostly children – who fell sick with a flu-like virus and then, just days later, were relegated to spend the rest of their lives in an iron lung.
For two years, researchers had been testing a vaccine that would hopefully be able to stop the spread of the virus and prevent the 45,000 infections each year that were keeping the nation in a chokehold. At the podium, Francis greeted the crowd and then proceeded to change the course of human history: The vaccine, he reported, was “safe, effective, and potent.” Widespread vaccination could begin in just a few weeks. The nightmare was over.
The road to success
Jonas Salk, a medical researcher and virologist who developed the vaccine with his own research team, would rightfully go down in history as the man who eradicated polio. (Today, wild poliovirus circulates in just two countries, Afghanistan and Pakistan – with only 140 cases reported in 2020.) But many people today forget that the widespread vaccination campaign that effectively ended wild polio across the globe would have never been possible without the human clinical trials that preceded it.
As with the COVID-19 vaccine, skepticism and misinformation around the polio vaccine abounded. But even more pervasive than the skepticism was fear. The consequences of polio had arguably never been more visible.
The road to human clinical trials – and the resulting vaccine – was a long one. In 1938, President Franklin Delano Roosevelt launched the National Foundation for Infantile Paralysis in order to raise funding for research and development of a polio vaccine. (Today, we know this organization as the March of Dimes.) A polio survivor himself, Roosevelt elevated awareness and prevention into the national spotlight, even more so than it had been previously. Raising funds for a safe and effective polio vaccine became a cornerstone of his presidency – and the funds raked in by his foundation went primarily to Salk to fund his research.
The Trials Begin
Salk’s vaccine, which included an inactivated (killed) polio virus, was promising – but now the researchers needed test subjects to make global vaccination a possibility. Because the aim of the vaccine was to prevent paralytic polio, researchers decided that they had to test the vaccine in the population that was most vulnerable to paralysis – young children. And, because the rate of paralysis was so low even among children, the team required many children to collect enough data. Francis, who led the trial to evaluate Salk’s vaccine, began the process of recruiting more than one million school-aged children between the ages of six and nine in 272 counties that had the highest incidence of the disease. The participants were nicknamed the “Polio Pioneers.”
Double-blind, placebo-based trials were considered the “gold standard” of epidemiological research back in Francis's day - and they remain the best approach we have today. These rigorous scientific studies are designed with two participant groups in mind. One group, called the test group, receives the experimental treatment (such as a vaccine); the other group, called the control, receives an inactive treatment known as a placebo. The researchers then compare the effects of the active treatment against the effects of the placebo, and every researcher is “blinded” as to which participants receive what treatment. That way, the results aren’t tainted by any possible biases.
But the study was controversial in that only some of the individual field trials at the county and state levels had a placebo group. Researchers described this as a “calculated risk,” meaning that while there were risks involved in giving the vaccine to a large number of children, the bigger risk was the potential paralysis or death that could come with being infected by polio. In all, just 200,000 children across the US received a placebo treatment, while an additional 725,000 children acted as observational controls – in other words, researchers monitored them for signs of infection, but did not give them any treatment.
As with the COVID-19 vaccine, skepticism and misinformation around the polio vaccine abounded. But even more pervasive than the skepticism was fear. President Roosevelt, who had made many public and televised appearances in a wheelchair, served as a perpetual reminder of the consequences of polio, as an infection at age 39 had rendered him permanently unable to walk. The consequences of polio had arguably never been more visible, and parents signed up their children in droves to participate in the study and offer them protection.
The Polio Pioneer Legacy
In a little less than a year, roughly half a million children received a dose of Salk’s polio vaccine. While plenty of children were hesitant to get the shot, many former participants still remember the fear surrounding the disease. One former participant, a Polio Pioneer named Debbie LaCrosse, writes of her experience: “There was no discussion, no listing of pros and cons. No amount of concern over possible side effects or other unknowns associated with a new vaccine could compare to the terrifying threat of polio.” For their participation, each kid received a certificate – and sometimes a pin – with the words “Polio Pioneer” emblazoned across the front.
When Francis announced the results of the trial on April 12, 1955, people did more than just breathe a sigh of relief – they openly celebrated, ringing church bells and flooding into the streets to embrace. Salk, who had become the face of the vaccine at that point, was instantly hailed as a national hero – and teachers around the country had their students to write him ‘thank you’ notes for his years of diligent work.
But while Salk went on to win national acclaim – even accepting the Presidential Medal of Freedom for his work on the polio vaccine in 1977 – his success was due in no small part to the children (and their parents) who took a risk in order to advance medical science. And that risk paid off: By the early 1960s, the yearly cases of polio in the United States had gone down to just 910. Where before the vaccine polio had caused around 15,000 cases of paralysis each year, only ten cases of paralysis were recorded in the entire country throughout the 1970s. And in 1979, the virus that once shuttered entire towns was declared officially eradicated in this country. Thanks to the efforts of these brave pioneers, the nation – along with the majority of the world – remains free of polio even today.
Why you should (virtually) care
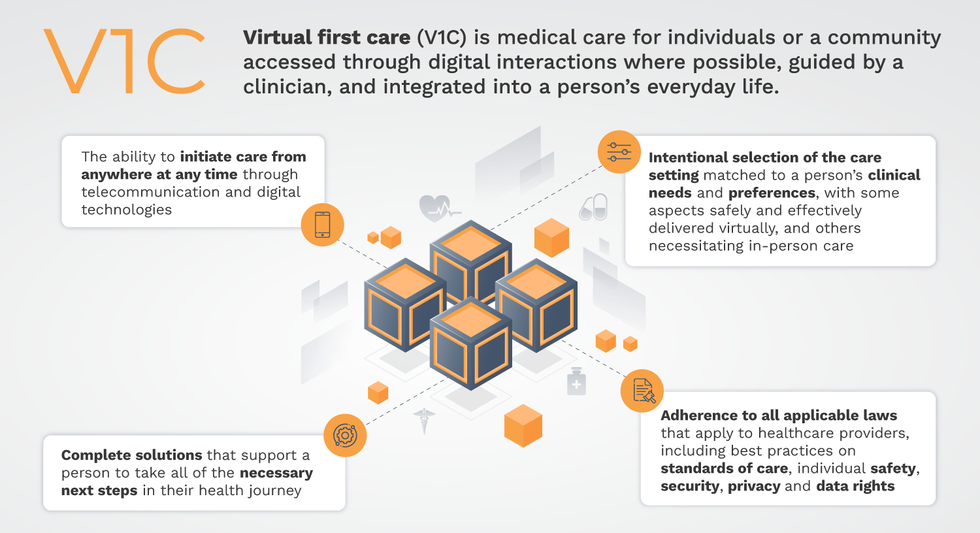
Virtual-first care, or V1C, could increase the quality of healthcare and make it more patient-centric by letting patients combine in-person visits with virtual options such as video for seeing their care providers.
As the pandemic turns endemic, healthcare providers have been eagerly urging patients to return to their offices to enjoy the benefits of in-person care.
But wait.
The last two years have forced all sorts of organizations to be nimble, adaptable and creative in how they work, and this includes healthcare providers’ efforts to maintain continuity of care under the most challenging of conditions. So before we go back to “business as usual,” don’t we owe it to those providers and ourselves to admit that business as usual did not work for most of the people the industry exists to help? If we’re going to embrace yet another period of change – periods that don’t happen often in our complex industry – shouldn’t we first stop and ask ourselves what we’re trying to achieve?
Certainly, COVID has shown that telehealth can be an invaluable tool, particularly for patients in rural and underserved communities that lack access to specialty care. It’s also become clear that many – though not all – healthcare encounters can be effectively conducted from afar. That said, the telehealth tactics that filled the gap during the pandemic were largely stitched together substitutes for existing visit-based workflows: with offices closed, patients scheduled video visits for help managing the side effects of their blood pressure medications or to see their endocrinologist for a quarterly check-in. Anyone whose children slogged through the last year or two of remote learning can tell you that simply virtualizing existing processes doesn’t necessarily improve the experience or the outcomes!
But what if our approach to post-pandemic healthcare came from a patient-driven perspective? We have a fleeting opportunity to advance a care model centered on convenient and equitable access that first prioritizes good outcomes, then selects approaches to care – and locations – tailored to each patient. Using the example of education, imagine how effective it would be if each student, regardless of their school district and aptitude, received such individualized attention.
That’s the idea behind virtual-first care (V1C), a new care model centered on convenient, customized, high-quality care that integrates a full suite of services tailored directly to patients’ clinical needs and preferences. This package includes asynchronous communication such as texting; video and other live virtual modes; and in-person options.
V1C goes beyond what you might think of as standard “telehealth” by using evidence-based protocols and tools that include traditional and digital therapeutics and testing, personalized care plans, dynamic patient monitoring, and team-based approaches to care. This could include spit kits mailed for laboratory tests and complementing clinical care with health coaching. V1C also replaces some in-person exams with ongoing monitoring, using sensors for more ‘whole person’ care.
Amidst all this momentum, we have the opportunity to rethink the goals of healthcare innovation, but that means bringing together key stakeholders to demonstrate that sustainable V1C can redefine healthcare.
Established V1C healthcare providers such as Omada, Headspace, and Heartbeat Health, as well as emerging market entrants like Oshi, Visana, and Wellinks, work with a variety of patients who have complicated long-term conditions such as diabetes, heart failure, gastrointestinal illness, endometriosis, and COPD. V1C is comprehensive in ways that are lacking in digital health and its other predecessors: it has the potential to integrate multiple data streams, incorporate more frequent touches and check-ins over time, and manage a much wider range of chronic health conditions, improving lives and reducing disease burden now and in the future.
Recognizing the pandemic-driven interest in virtual care, significant energy and resources are already flowing fast toward V1C. Some of the world’s largest innovators jumped into V1C early on: Verily, Alphabet’s Life Sciences Company, launched Onduo in 2016 to disrupt the diabetes healthcare market, and is now well positioned to scale its solutions. Major insurers like Aetna and United now offer virtual-first plans to members, responding as organizations expand virtual options for employees. Amidst all this momentum, we have the opportunity to rethink the goals of healthcare innovation, but that means bringing together key stakeholders to demonstrate that sustainable V1C can redefine healthcare.
That was the immediate impetus for IMPACT, a consortium of V1C companies, investors, payers and patients founded last year to ensure access to high-quality, evidence-based V1C. Developed by our team at the Digital Medicine Society (DiMe) in collaboration with the American Telemedicine Association (ATA), IMPACT has begun to explore key issues that include giving patients more integrated experiences when accessing both virtual and brick-and-mortar care.
Digital Medicine Society
V1C is not, nor should it be, virtual-only care. In this new era of hybrid healthcare, success will be defined by how well providers help patients navigate the transitions. How do we smoothly hand a patient off from an onsite primary care physician to, say, a virtual cardiologist? How do we get information from a brick-and-mortar to a digital portal? How do you manage dataflow while still staying HIPAA compliant? There are many complex regulatory implications for these new models, as well as an evolving landscape in terms of privacy, security and interoperability. It will be no small task for groups like IMPACT to determine the best path forward.
None of these factors matter unless the industry can recruit and retain clinicians. Our field is facing an unprecedented workforce crisis. Traditional healthcare is making clinicians miserable, and COVID has only accelerated the trend of overworked, disenchanted healthcare workers leaving in droves. Clinicians want more interactions with patients, and fewer with computer screens – call it “More face time, less FaceTime.” No new model will succeed unless the industry can more efficiently deploy its talent – arguably its most scarce and precious resource. V1C can help with alleviating the increasing burden and frustration borne by individual physicians in today’s status quo.
In healthcare, new technological approaches inevitably provoke no shortage of skepticism. Past lessons from Silicon Valley-driven fixes have led to understandable cynicism. But V1C is a different breed of animal. By building healthcare around the patient, not the clinic, V1C can make healthcare work better for patients, payers and providers. We’re at a fork in the road: we can revert back to a broken sick-care system, or dig in and do the hard work of figuring out how this future-forward healthcare system gets financed, organized and executed. As a field, we must find the courage and summon the energy to embrace this moment, and make it a moment of change.

