Researchers Behaving Badly: Known Frauds Are "the Tip of the Iceberg"

Disgraced stem cell researcher and celebrity surgeon Paolo Macchiarini.
Last week, the whistleblowers in the Paolo Macchiarini affair at Sweden's Karolinska Institutet went on the record here to detail the retaliation they suffered for trying to expose a star surgeon's appalling research misconduct.
Scientific fraud of the type committed by Macchiarini is rare, but studies suggest that it's on the rise.
The whistleblowers had discovered that in six published papers, Macchiarini falsified data, lied about the condition of patients and circumvented ethical approvals. As a result, multiple patients suffered and died. But Karolinska turned a blind eye for years.
Scientific fraud of the type committed by Macchiarini is rare, but studies suggest that it's on the rise. Just this week, for example, Retraction Watch and STAT together broke the news that a Harvard Medical School cardiologist and stem cell researcher, Piero Anversa, falsified data in a whopping 31 papers, which now have to be retracted. Anversa had claimed that he could regenerate heart muscle by injecting bone marrow cells into damaged hearts, a result that no one has been able to duplicate.
A 2009 study published in the Public Library of Science (PLOS) found that about two percent of scientists admitted to committing fabrication, falsification or plagiarism in their work. That's a small number, but up to one third of scientists admit to committing "questionable research practices" that fall into a gray area between rigorous accuracy and outright fraud.
These dubious practices may include misrepresentations, research bias, and inaccurate interpretations of data. One common questionable research practice entails formulating a hypothesis after the research is done in order to claim a successful premise. Another highly questionable practice that can shape research is ghost-authoring by representatives of the pharmaceutical industry and other for-profit fields. Still another is gifting co-authorship to unqualified but powerful individuals who can advance one's career. Such practices can unfairly bolster a scientist's reputation and increase the likelihood of getting the work published.
The above percentages represent what scientists admit to doing themselves; when they evaluate the practices of their colleagues, the numbers jump dramatically. In a 2012 study published in the Journal of Research in Medical Sciences, researchers estimated that 14 percent of other scientists commit serious misconduct, while up to 72 percent engage in questionable practices. While these are only estimates, the problem is clearly not one of just a few bad apples.
In the PLOS study, Daniele Fanelli says that increasing evidence suggests the known frauds are "just the 'tip of the iceberg,' and that many cases are never discovered" because fraud is extremely hard to detect.
Essentially everyone wants to be associated with big breakthroughs, and they may overlook scientifically shaky foundations when a major advance is claimed.
In addition, it's likely that most cases of scientific misconduct go unreported because of the high price of whistleblowing. Those in the Macchiarini case showed extraordinary persistence in their multi-year campaign to stop his deadly trachea implants, while suffering serious damage to their careers. Such heroic efforts to unmask fraud are probably rare.
To make matters worse, there are numerous players in the scientific world who may be complicit in either committing misconduct or covering it up. These include not only primary researchers but co-authors, institutional executives, journal editors, and industry leaders. Essentially everyone wants to be associated with big breakthroughs, and they may overlook scientifically shaky foundations when a major advance is claimed.
Another part of the problem is that it's rare for students in science and medicine to receive an education in ethics. And studies have shown that older, more experienced and possibly jaded researchers are more likely to fudge results than their younger, more idealistic colleagues.
So, given the steep price that individuals and institutions pay for scientific misconduct, what compels them to go down that road in the first place? According to the JRMS study, individuals face intense pressures to publish and to attract grant money in order to secure teaching positions at universities. Once they have acquired positions, the pressure is on to keep the grants and publishing credits coming in order to obtain tenure, be appointed to positions on boards, and recruit flocks of graduate students to assist in research. And not to be underestimated is the human ego.
Paolo Macchiarini is an especially vivid example of a scientist seeking not only fortune, but fame. He liberally (and falsely) claimed powerful politicians and celebrities, even the Pope, as patients or admirers. He may be an extreme example, but we live in an age of celebrity scientists who bring huge amounts of grant money and high prestige to the institutions that employ them.
The media plays a significant role in both glorifying stars and unmasking frauds. In the Macchiarini scandal, the media first lifted him up, as in NBC's laudatory documentary, "A Leap of Faith," which painted him as a kind of miracle-worker, and then brought him down, as in the January 2016 documentary, "The Experiments," which chronicled the agonizing death of one of his patients.
Institutions can also play a crucial role in scientific fraud by putting more emphasis on the number and frequency of papers published than on their quality. The whole course of a scientist's career is profoundly affected by something called the h-index. This is a number based on both the frequency of papers published and how many times the papers are cited by other researchers. Raising one's ranking on the h-index becomes an overriding goal, sometimes eclipsing the kind of patient, time-consuming research that leads to true breakthroughs based on reliable results.
Universities also create a high-pressured environment that encourages scientists to cut corners. They, too, place a heavy emphasis on attracting large monetary grants and accruing fame and prestige. This can lead them, just as it led Karolinska, to protect a star scientist's sloppy or questionable research. According to Dr. Andrew Rosenberg, who is director of the Center for Science and Democracy at the U.S.-based Union of Concerned Scientists, "Karolinska defended its investment in an individual as opposed to the long-term health of the institution. People were dying, and they should have outsourced the investigation from the very beginning."
Having institutions investigate their own practices is a conflict of interest from the get-go, says Rosenberg.
Scientists, universities, and research institutions are also not immune to fads. "Hot" subjects attract grant money and confer prestige, incentivizing scientists to shift their research priorities in a direction that garners more grants. This can mean neglecting the scientist's true area of expertise and interests in favor of a subject that's more likely to attract grant money. In Macchiarini's case, he was allegedly at the forefront of the currently sexy field of regenerative medicine -- a field in which Karolinska was making a huge investment.
The relative scarcity of resources intensifies the already significant pressure on scientists. They may want to publish results rapidly, since they face many competitors for limited grant money, academic positions, students, and influence. The scarcity means that a great many researchers will fail while only a few succeed. Once again, the temptation may be to rush research and to show it in the most positive light possible, even if it means fudging or exaggerating results.
Though the pressures facing scientists are very real, the problem of misconduct is not inevitable.
Intense competition can have a perverse effect on researchers, according to a 2007 study in the journal Science of Engineering and Ethics. Not only does it place undue pressure on scientists to succeed, it frequently leads to the withholding of information from colleagues, which undermines a system in which new discoveries build on the previous work of others. Researchers may feel compelled to withhold their results because of the pressure to be the first to publish. The study's authors propose that more investment in basic research from governments could alleviate some of these competitive pressures.
Scientific journals, although they play a part in publishing flawed science, can't be expected to investigate cases of suspected fraud, says the German science blogger Leonid Schneider. Schneider's writings helped to expose the Macchiarini affair.
"They just basically wait for someone to retract problematic papers," he says.
He also notes that, while American scientists can go to the Office of Research Integrity to report misconduct, whistleblowers in Europe have no external authority to whom they can appeal to investigate cases of fraud.
"They have to go to their employer, who has a vested interest in covering up cases of misconduct," he says.
Science is increasingly international. Major studies can include collaborators from several different countries, and he suggests there should be an international body accessible to all researchers that will investigate suspected fraud.
Ultimately, says Rosenberg, the scientific system must incorporate trust. "You trust co-authors when you write a paper, and peer reviewers at journals trust that scientists at research institutions like Karolinska are acting with integrity."
Without trust, the whole system falls apart. It's the trust of the public, an elusive asset once it has been betrayed, that science depends upon for its very existence. Scientific research is overwhelmingly financed by tax dollars, and the need for the goodwill of the public is more than an abstraction.
The Macchiarini affair raises a profound question of trust and responsibility: Should multiple co-authors be held responsible for a lead author's misconduct?
Karolinska apparently believes so. When the institution at last owned up to the scandal, it vindictively found Karl Henrik-Grinnemo, one of the whistleblowers, guilty of scientific misconduct as well. It also designated two other whistleblowers as "blameworthy" for their roles as co-authors of the papers on which Macchiarini was the lead author.
As a result, the whistleblowers' reputations and employment prospects have become collateral damage. Accusations of research misconduct can be a career killer. Research grants dry up, employment opportunities evaporate, publishing becomes next to impossible, and collaborators vanish into thin air.
Grinnemo contends that co-authors should only be responsible for their discrete contributions, not for the data supplied by others.
"Different aspects of a paper are highly specialized," he says, "and that's why you have multiple authors. You cannot go through every single bit of data because you don't understand all the parts of the article."
This is especially true in multidisciplinary, translational research, where there are sometimes 20 or more authors. "You have to trust co-authors, and if you find something wrong you have to notify all co-authors. But you couldn't go through everything or it would take years to publish an article," says Grinnemo.
Though the pressures facing scientists are very real, the problem of misconduct is not inevitable. Along with increased support from governments and industry, a change in academic culture that emphasizes quality over quantity of published studies could help encourage meritorious research.
But beyond that, trust will always play a role when numerous specialists unite to achieve a common goal: the accumulation of knowledge that will promote human health, wealth, and well-being.
[Correction: An earlier version of this story mistakenly credited The New York Times with breaking the news of the Anversa retractions, rather than Retraction Watch and STAT, which jointly published the exclusive on October 14th. The piece in the Times ran on October 15th. We regret the error.]
An astronaut peers through a portal in outer space.
What if people could just survive on sunlight like plants?
The admittedly outlandish question occurred to me after reading about how climate change will exacerbate drought, flooding, and worldwide food shortages. Many of these problems could be eliminated if human photosynthesis were possible. Had anyone ever tried it?
Extreme space travel exists at an ethically unique spot that makes human experimentation much more palatable.
I emailed Sidney Pierce, professor emeritus in the Department of Integrative Biology at the University of South Florida, who studies a type of sea slug, Elysia chlorotica, that eats photosynthetic algae, incorporating the algae's key cell structure into itself. It's still a mystery how exactly a slug can operate the part of the cell that converts sunlight into energy, which requires proteins made by genes to function, but the upshot is that the slugs can (and do) live on sunlight in-between feedings.
Pierce says he gets questions about human photosynthesis a couple of times a year, but it almost certainly wouldn't be worth it to try to develop the process in a human. "A high-metabolic rate, large animal like a human could probably not survive on photosynthesis," he wrote to me in an email. "The main reason is a lack of surface area. They would either have to grow leaves or pull a trailer covered with them."
In short: Plants have already exploited the best tricks for subsisting on photosynthesis, and unless we want to look and act like plants, we won't have much success ourselves. Not that it stopped Pierce from trying to develop human photosynthesis technology anyway: "I even tried to sell it to the Navy back in the day," he told me. "Imagine photosynthetic SEALS."
It turns out, however, that while no one is actively trying to create photosynthetic humans, scientists are considering the ways humans might need to change to adapt to future environments, either here on the rapidly changing Earth or on another planet. Rice University biologist Scott Solomon has written an entire book, Future Humans, in which he explores the environmental pressures that are likely to influence human evolution from this point forward. On Earth, Solomon says, infectious disease will remain a major driver of change. As for Mars, the big two are lower gravity and radiation, the latter of which bombards the Martian surface constantly because the planet has no magnetosphere.
Although he considers this example "pretty out there," Solomon says one possible solution to Mars' magnetic assault could leave humans not photosynthetic green, but orange, thanks to pigments called carotenoids that are responsible for the bright hues of pumpkins and carrots.
"Carotenoids protect against radiation," he says. "Usually only plants and microbes can produce carotenoids, but there's at least one kind of insect, a particular type of aphid, that somehow acquired the gene for making carotenoids from a fungus. We don't exactly know how that happened, but now they're orange... I view that as an example of, hey, maybe humans on Mars will evolve new kinds of pigmentation that will protect us from the radiation there."
We could wait for an orange human-producing genetic variation to occur naturally, or with new gene editing techniques such as CRISPR-Cas9, we could just directly give astronauts genetic advantages such as carotenoid-producing skin. This may not be as far-off as it sounds: Extreme space travel exists at an ethically unique spot that makes human experimentation much more palatable. If an astronaut already plans to subject herself to the enormous experiment of traveling to, and maybe living out her days on, a dangerous and faraway planet, do we have any obligation to provide all the protection we can?
Probably the most vocal person trying to figure out what genetic protections might help astronauts is Cornell geneticist Chris Mason. His lab has outlined a 10-phase, 500-year plan for human survival, starting with the comparatively modest goal of establishing which human genes are not amenable to change and should be marked with a "Do not disturb" sign.
To be clear, Mason is not actually modifying human beings. Instead, his lab has studied genes in radiation-resistant bacteria, such as the Deinococcus genus. They've expressed proteins called DSUP from tardigrades, tiny water bears that can survive in space, in human cells. They've looked into p53, a gene that is overexpressed in elephants and seems to protect them from cancer. They also developed a protocol to work on the NASA twin study comparing astronauts Scott Kelly, who spent a year aboard the International Space Station, and his brother Mark, who did not, to find out what effects space tends to have on genes in the first place.
In a talk he gave in December, Mason reported that 8.7 percent of Scott Kelly's genes—mostly those associated with immune function, DNA repair, and bone formation—did not return to normal after the astronaut had been home for six months. "Some of these space genes, we could engineer them, activate them, have them be hyperactive when you go to space," he said in that same talk. "When we think about having the hubris to go to a faraway planet...it seems like an almost impossible idea….but I really like people and I want us to survive for a long time, and this is the first step on the stairwell to survive out of the solar system."
What is the most important ability we could give our future selves through science?
There are others performing studies to figure out what capabilities we might bestow on the future-proof superhuman, but none of them are quite as extreme as photosynthesis (although all of them are useful). At Harvard, geneticist George Church wants to engineer cells to be resistant to viruses, such as the common cold and HIV. At Columbia, synthetic biologist Harris Wang is addressing self-sufficient humans more directly—trying to spur kidney cells to produce amino acids that are normally only available from diet.
But perhaps Future Humans author Scott Solomon has the most radical idea. I asked him a version of the classic What would be your superhero power? question: What does he see as the most important ability we could give our future selves through science?
"The empathy gene," he said. "The ability to put yourself in someone else's shoes and see the world as they see it. I think it would solve a lot of our problems."
A model of a human kidney.
Science's dream of creating perfect custom organs on demand as soon as a patient needs one is still a long way off. But tiny versions are already serving as useful research tools and stepping stones toward full-fledged replacements.
Although organoids cannot yet replace kidneys, they are invaluable tools for research.
The Lowdown
Australian researchers have grown hundreds of mini human kidneys in the past few years. Known as organoids, they function much like their full-grown counterparts, minus a few features due to a lack of blood supply.
Cultivated in a petri dish, these kidneys are still a shadow of their human counterparts. They grow no larger than one-sixth of an inch in diameter; fully developed organs are up to five inches in length. They contain no more than a few dozen nephrons, the kidney's individual blood-filtering unit, whereas a fully-grown kidney has about 1 million nephrons. And the dish variety live for just a few weeks.
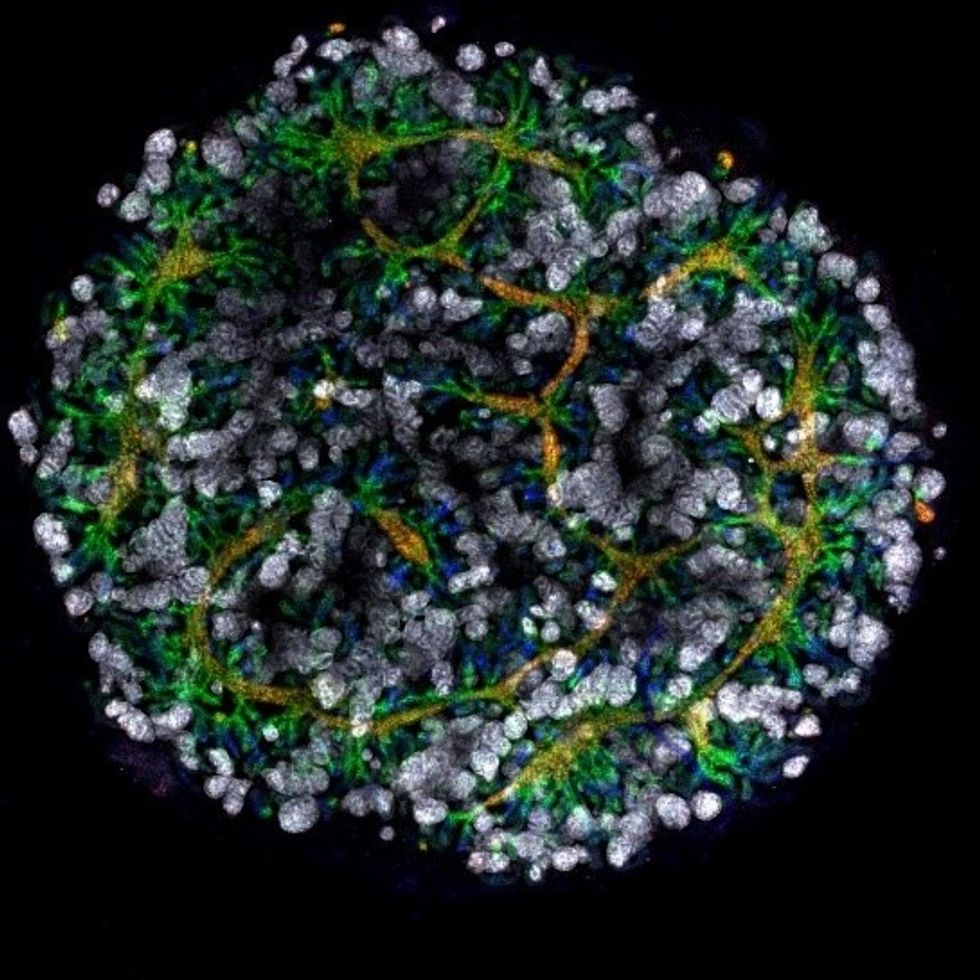
An organoid kidney created by the Murdoch Children's Institute in Melbourne, Australia.
Photo Credit: Shahnaz Khan.
But Melissa Little, head of the kidney research laboratory at the Murdoch Children's Institute in Melbourne, says these organoids are invaluable tools for research. Although renal failure is rare in children, more than half of those who suffer from such a disorder inherited it.
The mini kidneys enable scientists to better understand the progression of such disorders because they can be grown with a patient's specific genetic condition.
Mature stem cells can be extracted from a patient's blood sample and then reprogrammed to become like embryonic cells, able to turn into any type of cell in the body. It's akin to walking back the clock so that the cells regain unlimited potential for development. (The Japanese scientist who pioneered this technique was awarded the Nobel Prize in 2012.) These "induced pluripotent stem cells" can then be chemically coaxed to grow into mini kidneys that have the patient's genetic disorder.
"The (genetic) defects are quite clear in the organoids, and they can be monitored in the dish," Little says. To date, her research team has created organoids from 20 different stem cell lines.
Medication regimens can also be tested on the organoids, allowing specific tailoring for each patient. For now, such testing remains restricted to mice, but Little says it eventually will be done on human organoids so that the results can more accurately reflect how a given patient will respond to particular drugs.
Next Steps
Although these organoids cannot yet replace kidneys, Little says they may plug a huge gap in renal care by assisting in developing new treatments for chronic conditions. Currently, most patients with a serious kidney disorder see their options narrow to dialysis or organ transplantation. The former not only requires multiple sessions a week, but takes a huge toll on patient health.
Ten percent of older patients on dialysis die every year in the U.S. Aside from the physical trauma of organ transplantation, finding a suitable donor outside of a family member can be difficult.
"This is just another great example of the potential of pluripotent stem cells."
Meanwhile, the ongoing creation of organoids is supplying Little and her colleagues with enough information to create larger and more functional organs in the future. According to Little, researchers in the Netherlands, for example, have found that implanting organoids in mice leads to the creation of vascular growth, a potential pathway toward creating bigger and better kidneys.
And while Little acknowledges that creating a fully-formed custom organ is the ultimate goal, the mini organs are an important bridge step.
"This is just another great example of the potential of pluripotent stem cells, and I am just passionate to see it do some good."