This Revolutionary Medical Breakthrough Is Not a Treatment or a Cure

A doctor examines a patient to determine the cause of her illness.
What is a disease? This seemingly abstract and theoretical question is actually among the most practical questions in all of biomedicine. How patients are diagnosed, treated, managed and excused from various social and moral obligations hinges on the answer that is given. So do issues of how research is done and health care paid for. The question is also becoming one of the most problematic issues that those in health care will face in the next decade.
"The revolution in our understanding of the human genome, molecular biology, and genetics is creating a huge--if little acknowledged--shift in the understanding of what a disease is."
That is because the current conception of disease is undergoing a revolutionary change, fueled by progress in genetics and molecular biology. The consequences of this shift in the definition of disease promise to be as impactful as any other advance in biomedicine has ever been, which is admittedly saying a lot for what is in essence a conceptual change rather than one based on an empirical scientific advance.
For a long time, disease was defined by patient reports of feeling sick. It was not until the twentieth century that a shift occurred away from subjective reports of clusters of symptoms to defining diseases in terms of physiological states. Doctors began to realize that not all symptoms of fever represented the presence of the same disease. Flu got distinguished from malaria. Diseases such as hypertension, osteoporosis, cancer, lipidemia, silent myocardial infarction, retinopathy, blood clots and many others were recognized as not producing any or slight symptoms until suddenly the patient had a stroke or died.
The ability to assess both biology and biochemistry and to predict the consequences of subclinical pathological processes caused a distinction to be made between illness—what a person experiences—and disease—an underlying pathological process with a predictable course. Some conditions, such as Gulf War Syndrome, PTSD, many mental illnesses and fibromyalgia, remain controversial because no underlying pathological process has been found that correlates with them—a landmark criterion for diagnosing disease throughout most of the last century.
"Diseases for which no relationship had ever been posited are being lumped together due to common biochemical causal pathways...that are amenable to the same curative intervention."
The revolution in our understanding of the human genome, molecular biology, and genetics is creating a huge--if little acknowledged--shift in the understanding of what a disease is. A better understanding of the genetic and molecular roots of pathophysiology is leading to the reclassification of many familiar diseases. The test of disease is now not the pathophysiology but the presence of a gene, set of genes or molecular pathway that causes pathophysiology. Just as fever was differentiated into a multitude of diseases in the last century, cancer, cognitive impairment, addiction and many other diseases are being broken or split into many subkinds. And other diseases for which no relationship had ever been posited are being lumped together due to common biochemical causal pathways or the presence of similar dangerous biochemical products that are amenable to the same curative intervention, no matter how disparate the patients' symptoms or organic pathologies might appear.
We used to differentiate ovarian and breast cancers. Now we are thinking of them as outcomes of the same mutations in certain genes in the BRCA regions. They may eventually lump together as BRCA disease.
Other diseases such as familial amyloid polyneuropathy (FAP) which causes polyneuropathy and autonomic dysfunction are being split apart into new types or kinds. The disease is the product of mutations in the transthyretin gene. It was thought to be an autosomal dominant disease with symptomatic onset between 20-40 years of age. However, as genetic testing has improved, it has become clear that FAP's traditional clinical presentation represents a relatively small portion of those with FAP. Many patients with mutations in transthyretin — even mutations commonly seen in traditional FAP patients — do not fit the common clinical presentation. As the mutations begin to be understood, some people that were previously thought to have other polyneuropathies, such as chronic inflammatory demyelinating neuropathy, are now being rediagnosed with newly discovered variants of FAP.
"We are at the start of a major conceptual shift in how we organize the world of disease, and for that matter, health promotion."
Genome-wide association studies are beginning to find many links between diseases not thought to have any connection or association. For example some forms of diabetes, rheumatoid arthritis and thyroid disease may be the products of a small family of genetic mutations.
So why is this shift toward a genetic and molecular diagnostics likely to shake up medicine? One obvious way is that research projects may propose to recruit subjects not according to current standards of disease but on the basis of common genetic mutations or similar errors in biochemical pathways. It won't matter in a future study if subjects in a trial have what today might be termed nicotine addiction or Parkinsonism. If the molecular pathways producing the pathology are the same, then both groups might well wind up in the same trial of a drug.
In addition, what today look like common maladies—pancreatic cancer, severe depression, or acne, for example, could wind up being subdivided into so many highly differentiated versions of these conditions that each must be treated as what we now classify as a rare or ultra-rare disease. Unique biochemical markers or genetic messages may see many diseases broken into a huge number of distinct individual disease entities.
Patients may find that common genetic pathways or multiple effects from a single gene may create new alliances for fund-raising and advocacy. Groups fighting to cure mental and physical illnesses may wind up forgetting about their outward differences in the effort to alter genes or attack common protein markers.
Disease classification appears stable to us—until it isn't. And we are at the start of a major conceptual shift in how we organize the world of disease, and for that matter, health promotion. Classic reductionism, the view that all observable biological phenomena can be explained in terms of underlying chemical and physical principles, may turn out not to be true. But the molecular and genetic revolutions churning through medicine are illustrating that reductionism is going to have an enormous influence on disease classification. That is not a bad thing, but it is something that is going to take a lot to get used to.
A robot server, controlled remotely by a disabled worker, delivers drinks to patrons at the DAWN cafe in Tokyo.
A sleek, four-foot tall white robot glides across a cafe storefront in Tokyo’s Nihonbashi district, holding a two-tiered serving tray full of tea sandwiches and pastries. The cafe’s patrons smile and say thanks as they take the tray—but it’s not the robot they’re thanking. Instead, the patrons are talking to the person controlling the robot—a restaurant employee who operates the avatar from the comfort of their home.
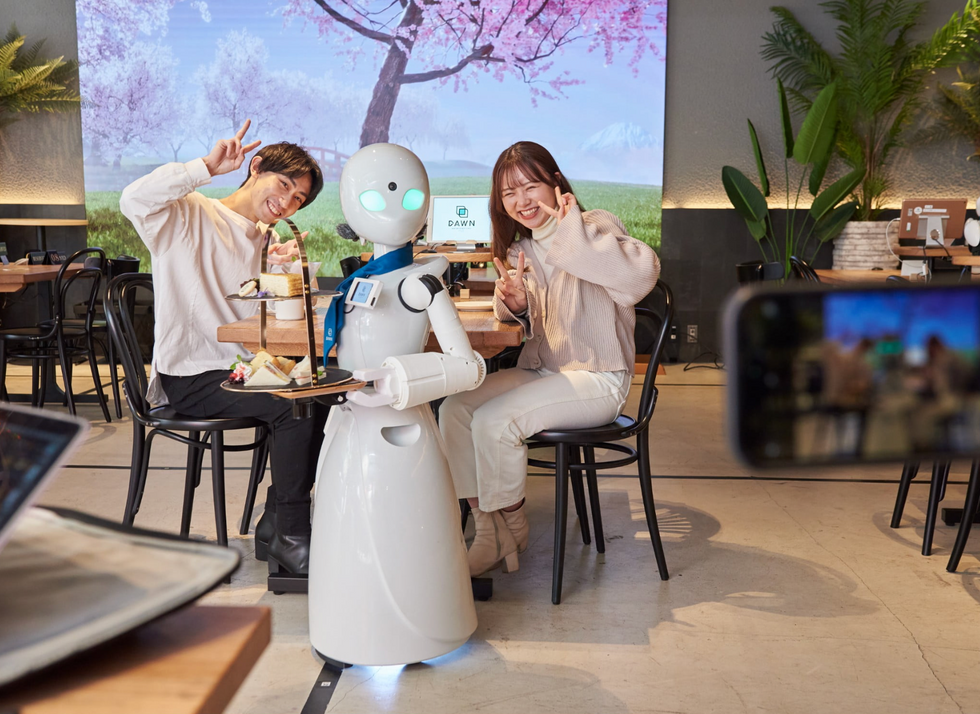
It’s a typical scene at DAWN, short for Diverse Avatar Working Network—a cafe that launched in Tokyo six years ago as an experimental pop-up and quickly became an overnight success. Today, the cafe is a permanent fixture in Nihonbashi, staffing roughly 60 remote workers who control the robots remotely and communicate to customers via a built-in microphone.
More than just a creative idea, however, DAWN is being hailed as a life-changing opportunity. The workers who control the robots remotely (known as “pilots”) all have disabilities that limit their ability to move around freely and travel outside their homes. Worldwide, an estimated 16 percent of the global population lives with a significant disability—and according to the World Health Organization, these disabilities give rise to other problems, such as exclusion from education, unemployment, and poverty.
These are all problems that Kentaro Yoshifuji, founder and CEO of Ory Laboratory, which supplies the robot servers at DAWN, is looking to correct. Yoshifuji, who was bedridden for several years in high school due to an undisclosed health problem, launched the company to help enable people who are house-bound or bedridden to more fully participate in society, as well as end the loneliness, isolation, and feelings of worthlessness that can sometimes go hand-in-hand with being disabled.
“It’s heartbreaking to think that [people with disabilities] feel they are a burden to society, or that they fear their families suffer by caring for them,” said Yoshifuji in an interview in 2020. “We are dedicating ourselves to providing workable, technology-based solutions. That is our purpose.”
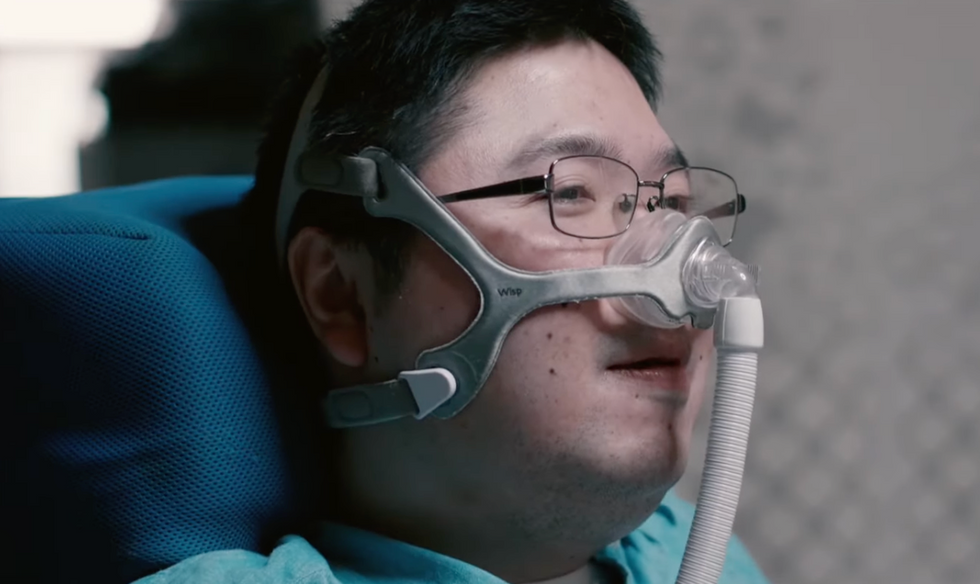
Shota, Kuwahara, a DAWN employee with muscular dystrophy, agrees. "There are many difficulties in my daily life, but I believe my life has a purpose and is not being wasted," he says. "Being useful, able to help other people, even feeling needed by others, is so motivational."
A woman receives a mammogram, which can detect the presence of tumors in a patient's breast.
When a patient is diagnosed with early-stage breast cancer, having surgery to remove the tumor is considered the standard of care. But what happens when a patient can’t have surgery?
Whether it’s due to high blood pressure, advanced age, heart issues, or other reasons, some breast cancer patients don’t qualify for a lumpectomy—one of the most common treatment options for early-stage breast cancer. A lumpectomy surgically removes the tumor while keeping the patient’s breast intact, while a mastectomy removes the entire breast and nearby lymph nodes.
Fortunately, a new technique called cryoablation is now available for breast cancer patients who either aren’t candidates for surgery or don’t feel comfortable undergoing a surgical procedure. With cryoablation, doctors use an ultrasound or CT scan to locate any tumors inside the patient’s breast. They then insert small, needle-like probes into the patient's breast which create an “ice ball” that surrounds the tumor and kills the cancer cells.
Cryoablation has been used for decades to treat cancers of the kidneys and liver—but only in the past few years have doctors been able to use the procedure to treat breast cancer patients. And while clinical trials have shown that cryoablation works for tumors smaller than 1.5 centimeters, a recent clinical trial at Memorial Sloan Kettering Cancer Center in New York has shown that it can work for larger tumors, too.
In this study, doctors performed cryoablation on patients whose tumors were, on average, 2.5 centimeters. The cryoablation procedure lasted for about 30 minutes, and patients were able to go home on the same day following treatment. Doctors then followed up with the patients after 16 months. In the follow-up, doctors found the recurrence rate for tumors after using cryoablation was only 10 percent.
For patients who don’t qualify for surgery, radiation and hormonal therapy is typically used to treat tumors. However, said Yolanda Brice, M.D., an interventional radiologist at Memorial Sloan Kettering Cancer Center, “when treated with only radiation and hormonal therapy, the tumors will eventually return.” Cryotherapy, Brice said, could be a more effective way to treat cancer for patients who can’t have surgery.
“The fact that we only saw a 10 percent recurrence rate in our study is incredibly promising,” she said.

