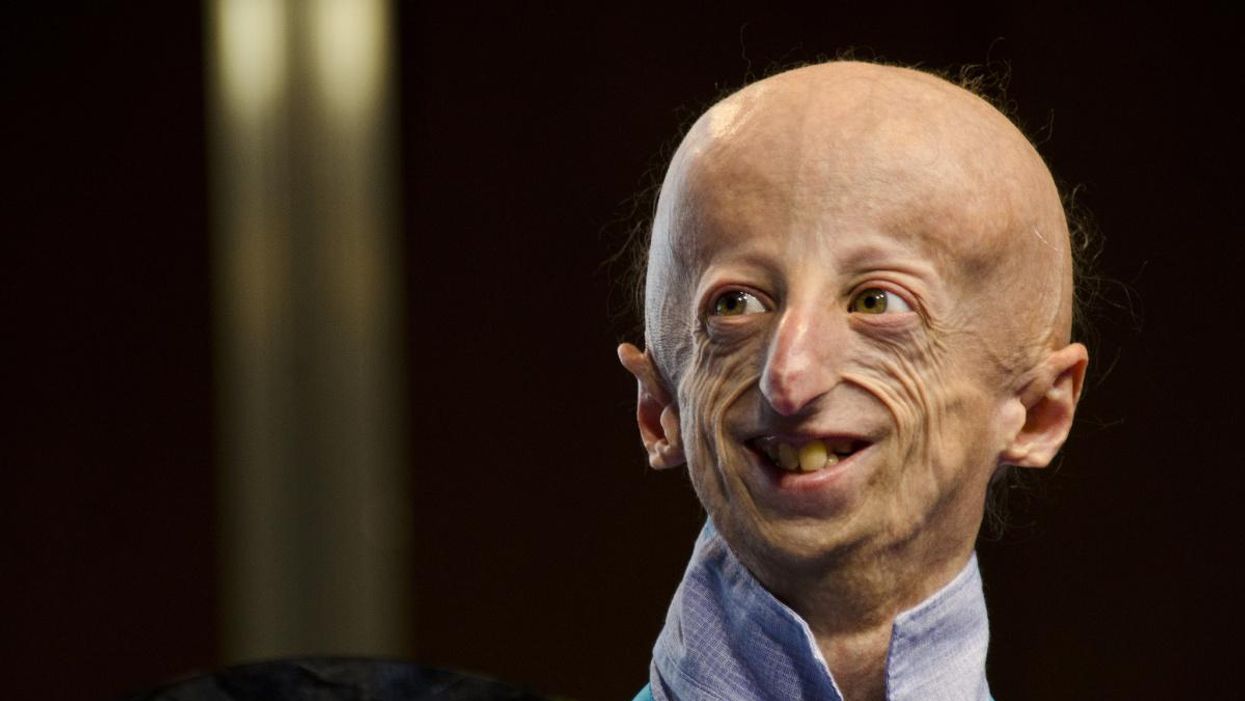
Paralyzed By Polio, This British Tea Broker Changed the Course Of Medical History Forever

Robin Cavendish in his special wheelchair with his son Jonathan in the 1960s.
In December 1958, on a vacation with his wife in Kenya, a 28-year-old British tea broker named Robin Cavendish became suddenly ill. Neither he nor his wife Diana knew it at the time, but Robin's illness would change the course of medical history forever.
Robin was rushed to a nearby hospital in Kenya where the medical staff delivered the crushing news: Robin had contracted polio, and the paralysis creeping up his body was almost certainly permanent. The doctors placed Robin on a ventilator through a tracheotomy in his neck, as the paralysis from his polio infection had rendered him unable to breathe on his own – and going off the average life expectancy at the time, they gave him only three months to live. Robin and Diana (who was pregnant at the time with their first child, Jonathan) flew back to England so he could be admitted to a hospital. They mentally prepared to wait out Robin's final days.
But Robin did something unexpected when he returned to the UK – just one of many things that would astonish doctors over the next several years: He survived. Diana gave birth to Jonathan in February 1959 and continued to visit Robin regularly in the hospital with the baby. Despite doctors warning that he would soon succumb to his illness, Robin kept living.
After a year in the hospital, Diana suggested something radical: She wanted Robin to leave the hospital and live at home in South Oxfordshire for as long as he possibly could, with her as his nurse. At the time, this suggestion was unheard of. People like Robin who depended on machinery to keep them breathing had only ever lived inside hospital walls, as the prevailing belief was that the machinery needed to keep them alive was too complicated for laypeople to operate. But Diana and Robin were up for the challenges – and the risks. Because his ventilator ran on electricity, if the house were to unexpectedly lose power, Diana would either need to restore power quickly or hand-pump air into his lungs to keep him alive.
Robin's wheelchair was not only the first of its kind; it became the model for the respiratory wheelchairs that people still use today.
In an interview as an adult, Jonathan Cavendish reflected on his parents' decision to live outside the hospital on a ventilator: "My father's mantra was quality of life," he explained. "He could have stayed in the hospital, but he didn't think that was as good of a life as he could manage. He would rather be two minutes away from death and living a full life."
After a few years of living at home, however, Robin became tired of being confined to his bed. He longed to sit outside, to visit friends, to travel – but had no way of doing so without his ventilator. So together with his friend Teddy Hall, a professor and engineer at Oxford University, the two collaborated in 1962 to create an entirely new invention: a battery-operated wheelchair prototype with a ventilator built in. With this, Robin could now venture outside the house – and soon the Cavendish family became famous for taking vacations. It was something that, by all accounts, had never been done before by someone who was ventilator-dependent. Robin and Hall also designed a van so that the wheelchair could be plugged in and powered during travel. Jonathan Cavendish later recalled a particular family vacation that nearly ended in disaster when the van broke down outside of Barcelona, Spain:
"My poor old uncle [plugged] my father's chair into the wrong socket," Cavendish later recalled, causing the electricity to short. "There was fire and smoke, and both the van and the chair ground to a halt." Johnathan, who was eight or nine at the time, his mother, and his uncle took turns hand-pumping Robin's ventilator by the roadside for the next thirty-six hours, waiting for Professor Hall to arrive in town and repair the van. Rather than being panicked, the Cavendishes managed to turn the vigil into a party. Townspeople came to greet them, bringing food and music, and a local priest even stopped by to give his blessing.
Robin had become a pioneer, showing the world that a person with severe disabilities could still have mobility, access, and a fuller quality of life than anyone had imagined. His mission, along with Hall's, then became gifting this independence to others like himself. Robin and Hall raised money – first from the Ernest Kleinwort Charitable Trust, and then from the British Department of Health – to fund more ventilator chairs, which were then manufactured by Hall's company, Littlemore Scientific Engineering, and given to fellow patients who wanted to live full lives at home. Robin and Hall used themselves as guinea pigs, testing out different models of the chairs and collaborating with scientists to create other devices for those with disabilities. One invention, called the Possum, allowed paraplegics to control things like the telephone and television set with just a nod of the head. Robin's wheelchair was not only the first of its kind; it became the model for the respiratory wheelchairs that people still use today.
Robin went on to enjoy a long and happy life with his family at their house in South Oxfordshire, surrounded by friends who would later attest to his "down-to-earth" personality, his sense of humor, and his "irresistible" charm. When he died peacefully at his home in 1994 at age 64, he was considered the world's oldest-living person who used a ventilator outside the hospital – breaking yet another barrier for what medical science thought was possible.
For Kids with Progeria, New Therapies May Offer Revolutionary Hope for a Longer Life
Sammy Basso is one of around 400 young people in the world with progeria.
Sammy Basso has some profound ideas about fate. As long as he has been alive, he has known he has minimal control over his own. His parents, however, had to transition from a world of unlimited possibility to one in which their son might not live to his 20s.
"I remember very clearly that day because Sammy was three years old," his mother says of the day a genetic counselor diagnosed Sammy with progeria. "It was a devastating day for me."
But to Sammy, he has always been himself: a smart kid, interested in science, a little smaller than his classmates, with one notable kink in his DNA. In one copy of the gene that codes for the protein Lamin A, Sammy has a T where there should be a C. The incorrect code creates a toxic protein called progerin, which destabilizes Sammy's cells and makes him age much faster than a person who doesn't have the mutation. The older he gets, the more he is in danger of strokes, heart failure, or a heart attack. "I am okay with my situation," he says from his home in Tezze sul Brenta, Italy. "But I think, yes, fate has a great role in my life."
Just 400 or so people in the world live with progeria: The mutation that causes it usually arises de novo, or "of new," meaning that it is not inherited but happens spontaneously during gestation. The challenge, as with all rare diseases, is that few cases means few treatments.
"When we first started, there was absolutely nothing out there," says Leslie Gordon, a physician-researcher who co-founded the Progeria Research Foundation in 1999 after her own son, also named Sam, was diagnosed with the disease. "We knew we had to jumpstart the entire field, so we collected money through road races and special events and writing grants and all sorts of donors… I think the first year we raised $75,000, most of it from one donor."
"We have not only the possibility but the responsibility to make the world a better world, and also to make a body a better body."
By 2003, the foundation had collaborated with Francis Collins, a geneticist who is now director of the National Institutes of Health, to work out the genetic basis for progeria—that single mutation Sammy has. The discovery led to interest in lonafarnib, a drug that was already being used in cancer patients but could potentially operate downstream of the mutation, preventing the buildup of the defective progerin in the body. "We funded cellular studies to look at a lonafarnib in cells, mouse studies to look at lonafarnib in mouse models of progeria… and then we initiated the clinical trials," Gordon says.
Sammy Basso's family had gotten involved with the Progeria Research Foundation through their international patient registry, which maintains relationships with families in 49 countries. "We started to hear about lonafarnib in 2006 from Leslie Gordon," says Sammy's father, Amerigo Basso, with his son translating. "She told us about the lonafarnib. And we were very happy because for the first time we understood that there was something that could help our son and our lives." Amerigo used the Italian word speranza, which means hope.
Still, Sammy wasn't sure if lonafarnib was right for him. "Since when I was very young I thought that everything happens for a reason. So, in my mind, if God made me with progeria, there was a reason, and to try to heal from progeria was something wrong," he says. Gradually, his parents and doctors, and Leslie Gordon, convinced him otherwise. Sammy began to believe that God was also the force behind doctors, science, and research. "And so we have not only the possibility but the responsibility to make the world a better world, and also to make a body a better body," he says.

Sammy Basso and his parents.
Courtesy of Basso
Sammy began taking lonafarnib, with the Progeria Research Foundation intermittently flying him, and other international trial participants, to Boston for tests. He was immediately beset by some of the drug's more unpleasant side effects: Stomach problems, nausea, and vomiting. "The first period was absolutely the worst period of my life," he says.
At first, doctors prescribed other medicines for the side effects, but to Sammy it had as much effect as drinking water. He visited doctor after doctor, with some calling him weekly or even daily to ask how he was doing. Eventually the specialists decided that he should lower his dose, balancing his pain with the benefit of the drug. Sammy can't actually feel any positive effect of the lonafarnib, but his health measurements have improved relative to people with progeria who don't take it.
While they never completely disappeared, Sammy's side effects decreased to the point that he could live. Inspired by the research that led to lonafarnib, he went to university to study molecular biology. For his thesis work, he travelled to Spain to perform experiments on cells and on mice with progeria, learning how to use the gene-editing technique CRISPR-Cas9 to cut out the mutated bit of DNA. "I was so excited to participate in this study," Sammy says. He felt like his work could make a difference.
In 2018, the Progeria Research Foundation was hosting one of their biennial workshops when Francis Collins, the researcher who had located the mutation behind progeria 15 years earlier, got in touch with Leslie Gordon. "Francis called me and said, Hey, I just saw a talk by David Liu from the Broad [Institute]. And it was pretty amazing. He has been looking at progeria and has very early, but very exciting data… Do you have any spaces, any slots you could make in your program for late breaking news?"
Gordon found a spot, and David Liu came to talk about what was going on in his lab, which was an even more advanced treatment that led to mice with the progeria mutation living into their senior mouse years—substantially closer to a normal lifespan. Liu's lab had built on the idea of CRISPR-Cas9 to create a more elegant genetic process called base editing: Instead of chopping out mutated DNA, a scientist could chemically convert an incorrect DNA letter to the correct one, like the search and replace function in word processing software. Mice who had their Lamin-A mutations corrected this way lived more than twice as long as untreated animals.
Sammy was in the audience at Dr. Liu's talk. "When I heard about this base editing as a younger scientist, I thought that I was living in the future," he says. "When my parents had my diagnosis of progeria, the science knew very little information about DNA. And now we are talking about healing the DNA… It is incredible."
Lonafarnib (also called Zokinvy) was approved by the US Food and Drug Administration this past November. Sammy, now 25, still takes it, and still manages his side effects. With luck, the gift of a few extra years will act as a bridge until he can try Liu's revolutionary new gene treatment, which has not yet begun testing in humans. While Leslie Gordon warns that she's always wrong about things like this, she hopes to see the new base editing techniques in clinical trials in the next year or two. Sammy won't need to be convinced to try it this time; his thinking on fate has evolved since his first encounter with lonafarnib.
"I would be very happy to try it," he says. "I know that for a non-scientist it can be difficult to understand. Some people think that we are the DNA. We are not. The DNA is a part of us, and to correct it is to do what we are already doing—just better." In short, a gene therapy, while it may seem like science fiction, is no different from a pill. For Sammy, both are a new way to think about fate: No longer something that simply happens to him.
Want to Motivate Vaccinations? Message Optimism, Not Doom
There is a lot to be optimistic about regarding the new safe and highly effective vaccines, which are moving society closer toward the goal of close human contact once again.
After COVID-19 was declared a worldwide pandemic by the World Health Organization on March 11, 2020, life as we knew it altered dramatically and millions went into lockdown. Since then, most of the world has had to contend with masks, distancing, ventilation and cycles of lockdowns as surges flare up. Deaths from COVID-19 infection, along with economic and mental health effects from the shutdowns, have been devastating. The need for an ultimate solution -- safe and effective vaccines -- has been paramount.
On November 9, 2020 (just 8 months after the pandemic announcement), the press release for the first effective COVID-19 vaccine from Pfizer/BioNTech was issued, followed by positive announcements regarding the safety and efficacy of five other vaccines from Moderna, University of Oxford/AztraZeneca, Novavax, Johnson and Johnson and Sputnik V. The Moderna and Pfizer vaccines have earned emergency use authorization through the FDA in the United States and are being distributed. We -- after many long months -- are seeing control of the devastating COVID-19 pandemic glimmering into sight.
To be clear, these vaccine candidates for COVID-19, both authorized and not yet authorized, are highly effective and safe. In fact, across all trials and sites, all six vaccines were 100% effective in preventing hospitalizations and death from COVID-19.
All Vaccines' Phase 3 Clinical Data
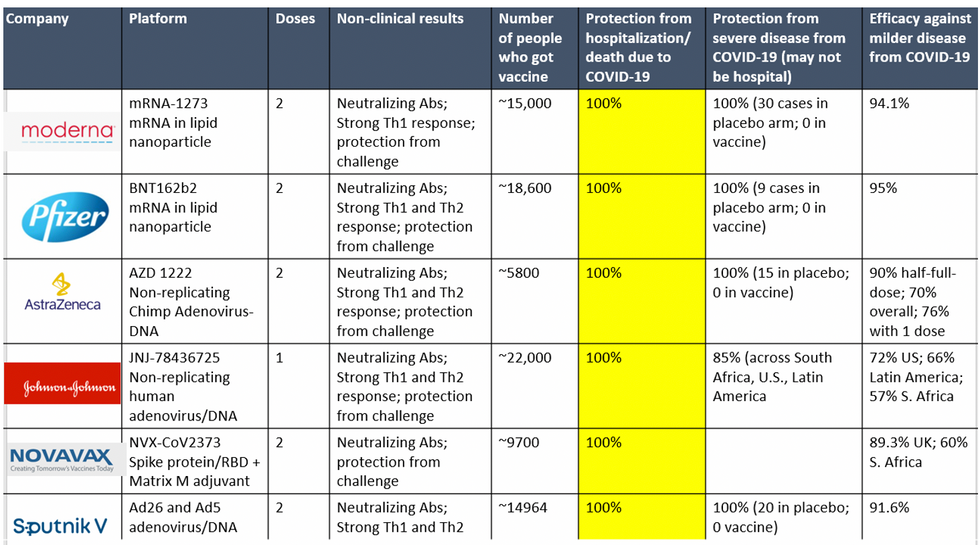
Complete protection against hospitalization and death from COVID-19 exhibited by all vaccines with phase 3 clinical trial data.
This astounding level of protection from SARS-CoV-2 from all vaccine candidates across multiple regions is likely due to robust T cell response from vaccination and will "defang" the virus from the concerns that led to COVID-19 restrictions initially: the ability of the virus to cause severe illness. This is a time of hope and optimism. After the devastating third surge of COVID-19 infections and deaths over the winter, we finally have an opportunity to stem the crisis – if only people readily accept the vaccines.
Amidst these incredible scientific advancements, however, public health officials and politicians have been pushing downright discouraging messaging. The ubiquitous talk of ongoing masks and distancing restrictions without any clear end in sight threatens to dampen uptake of the vaccines. It's imperative that we break down each concern and see if we can revitalize our public health messaging accordingly.
The first concern: we currently do not know if the vaccines block asymptomatic infection as well as symptomatic disease, since none of the phase 3 vaccine trials were set up to answer this question. However, there is biological plausibility that the antibodies and T-cell responses blocking symptomatic disease will also block asymptomatic infection in the nasal passages. IgG immunoglobulins (generated and measured by the vaccine trials) enter the nasal mucosa and systemic vaccinations generate IgA antibodies at mucosal surfaces. Monoclonal antibodies given to outpatients with COVID-19 hasten viral clearance from the airways.
Although it is prudent for those who are vaccinated to wear masks around the unvaccinated in case a slight risk of transmission remains, two fully vaccinated people can comfortably abandon masking around each other.
Moreover, data from the AztraZeneca trial (including in the phase 3 trial final results manuscript), where weekly self-swabbing was done by participants, and data from the Moderna trial, where a nasal swab was performed prior to the second dose, both showed risk reductions in asymptomatic infection with even a single dose. Finally, real-world data from a large Pfizer-based vaccine campaign in Israel shows a 50% reduction in infections (asymptomatic or symptomatic) after just the first dose.
Therefore, the likelihood of these vaccines blocking asymptomatic carriage, as well as symptomatic disease, is high. Although it is prudent for those who are vaccinated to wear masks around the unvaccinated in case a slight risk of transmission remains, two fully vaccinated people can comfortably abandon masking around each other. Moreover, as the percentage of vaccinated people increases, it will be increasingly untenable to impose restrictions on this group. Once herd immunity is reached, these restrictions can and should be abandoned altogether.
The second concern translating to "doom and gloom" messaging lately is around the identification of troubling new variants due to enhanced surveillance via viral sequencing. Four major variants circulating at this point (with others described in the past) are the B.1.1.7 variant ("UK variant"), B.1.351 ("South Africa variant), P.1. ("Brazil variant"), and the L452R variant identified in California. Although the UK variant is likely to be more transmissible, as is the South Africa variant, we have no reason to believe that masks, distancing and ventilation are ineffective against these variants.
Moreover, neutralizing antibody titers with the Pfizer and Moderna vaccines do not seem to be significantly reduced against the variants. Finally, although the Novavax 2-dose and Johnson and Johnson (J&J) 1-dose vaccines had lower rates of efficacy against moderate COVID-19 disease in South Africa, their efficacy against severe disease was impressively high. In fact J&J's vaccine still prevented 100% of hospitalizations and death from COVID-19. When combining both hospitalizations/deaths and severe symptoms managed at home, the J&J 1-dose vaccine was 85% protective across all three sites of the trial: the U.S., Latin America (including Brazil), and South Africa.
In South Africa, nearly all cases of COVID-19 (95%) were due to infection with the B.1.351 SARS-CoV-2 variant. Finally, since herd immunity does not rely on maximal immune responses among all individuals in a society, the Moderna/Pfizer/J&J vaccines are all likely to achieve that goal against variants. And thankfully, all of these vaccines can be easily modified to boost specifically against a new variant if needed (indeed, Moderna and Pfizer are already working on boosters against the prominent variants).
The third concern of some public health officials is that people will abandon all restrictions once vaccinated unless overly cautious messages are drilled into them. Indeed, the false idea that if you "give people an inch, they will take a mile" has been misinforming our messaging about mitigation since the beginning of the pandemic. For example, the very phrase "stay at home" with all of its non-applicability for essential workers and single individuals is stigmatizing and unrealistic for many. Instead, the message should have focused on how people can additively reduce their risks under different circumstances.
The public will be more inclined to trust health officials if those officials communicate with nuanced messages backed up by evidence, rather than with broad brushstrokes that shame. Therefore, we should be saying that "vaccinated people can be together with other vaccinated individuals without restrictions but must protect the unvaccinated with masks and distancing." And we can say "unvaccinated individuals should adhere to all current restrictions until vaccinated" without fear of misunderstandings. Indeed, this kind of layered advice has been communicated to people living with HIV and those without HIV for a long time (if you have HIV but partner does not, take these precautions; if both have HIV, you can do this, etc.).
Our heady progress in vaccine development, along with the incredible efficacy results of all of them, is unprecedented. However, we are at risk of undermining such progress if people balk at the vaccine because they don't believe it will make enough of a difference. One of the most critical messages we can deliver right now is that these vaccines will eventually free us from the restrictions of this pandemic. Let's use tiered messaging and clear communication to boost vaccine optimism and uptake, and get us to the goal of close human contact once again.