Podcast: Should Scientific Controversies Be Silenced?
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.

The recent Joe Rogan/Spotify controversy prompts the consideration of tough questions about expertise, trust, gatekeepers, and dissent.
The "Making Sense of Science" podcast features interviews with leading medical and scientific experts about the latest developments and the big ethical and societal questions they raise. This monthly podcast is hosted by journalist Kira Peikoff, founding editor of the award-winning science outlet Leaps.org.
The recent Joe Rogan/Spotify backlash over the misinformation presented in his recent episode on the Covid-19 vaccines raises some difficult and important bioethical questions for society: How can people know which experts to trust? What should big tech gatekeepers do about false claims promoted on their platforms? How should the scientific establishment respond to heterodox viewpoints from experts who disagree with the consensus? When is silencing of dissent merited, and when is it problematic? Journalist Kira Peikoff asks infectious disease physician and pandemic scholar Dr. Amesh Adalja to weigh in.

Dr. Amesh Adalja, Senior Scholar, Johns Hopkins Center for Health Security and an infectious disease physician
Listen to the Episode
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
Blood Donated from Recovered Coronavirus Patients May Soon Yield a Stopgap Treatment
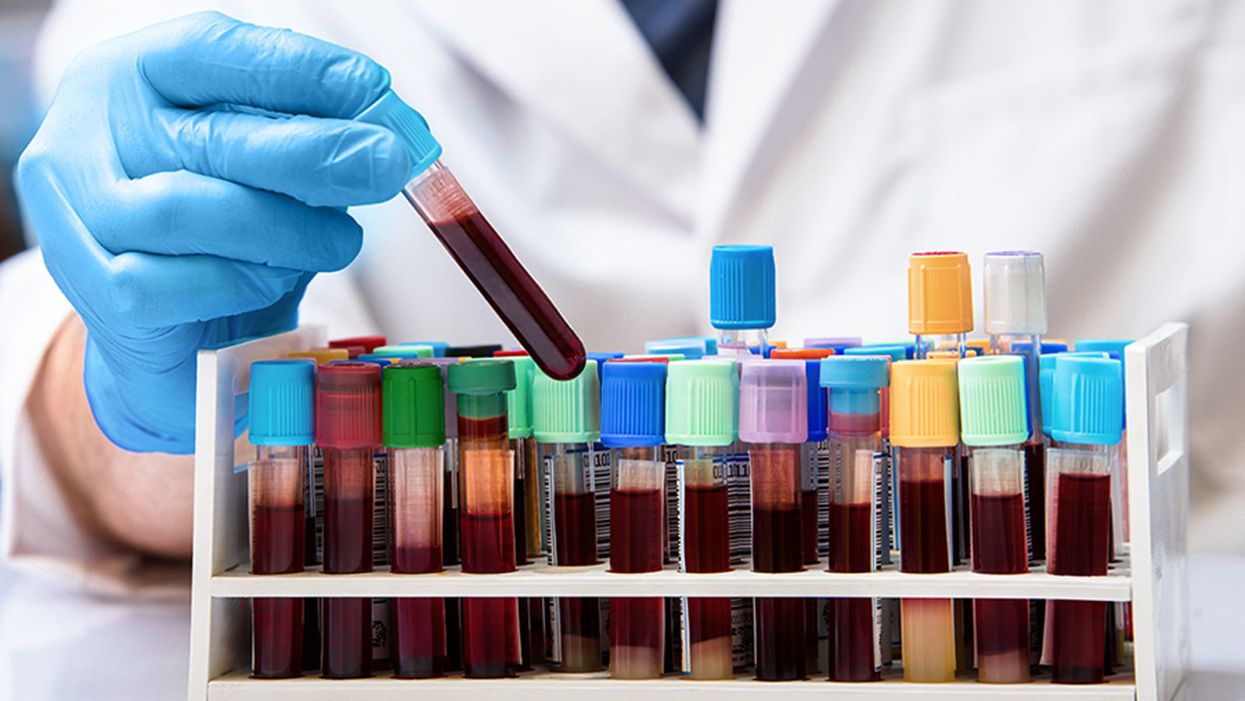
Antibodies from the blood of recovered patients is being tested as a stopgap treatment.
In October 1918, Lieutenant L.W. McGuire of the United States Navy sent a report to the American Journal of Public Health detailing a promising therapy that had already saved the lives of a number of officers suffering from pneumonia complications due to the Spanish influenza outbreak.
"These antibodies then become essentially drugs."
McGuire described how transfusions of blood from recovered patients – an idea which had first been trialed during a polio epidemic in 1916 – had led to rapid recovery in a series of severe pneumonia cases at a Naval Hospital in Massachusetts. "It is believed the serum has a decided influence in shortening the course of the disease, and lowering the mortality," he wrote.
Now more than a century on, this treatment – long forgotten in the western world - is once again coming to the fore during the current COVID-19 pandemic. With fatalities continuing to rise, and no vaccine expected for many months, experts are urging medical centers across the U.S. and Europe to initiate collaborations between critical care and transfusion services to offer this as an emergency treatment for those who need it most.
As of March 20, there are more than 90,000 individuals globally who have recovered from the disease. Some scientists believe that the blood of many of these people contains high levels of neutralizing antibodies that can kill the virus.
"These antibodies then become essentially drugs," said Arturo Casadevall, professor of Molecular Microbiology & Immunology at John Hopkins Bloomberg School of Public Health, who is currently co-ordinating a clinical trial of convalescent serum for COVID-19 involving 20 institutions across the US.
"We're talking about preparing a therapy right out of the serum of those that have recovered. It could also be used in patients who are already sick, but have not progressed to respiratory failure, to treat them before they enter intensive care units. That will provide a lot of support because there's a limited number of respirators and resources."
The first conclusive data on how the blood of recovered patients can help tackle COVID-19 is set to come out of China, where it was also used as an emergency treatment during the SARS and MERS outbreaks. On February 9, a severely ill patient in Wuhan was treated with convalescent serum and since then, hospitals across China have used the therapy on a total of 245 patients, with 91 reportedly showing an improvement in symptoms.
In China alone, more than 58,000 patients have now recovered from COVID-19. Casadevall said that last week the country shipped 90 tons of serum and plasma from these patients to Italy – the center of the pandemic in Europe – for emergency use.
Some of the first people to be treated are likely to be doctors and nurses in hospitals who are most at risk of exposure.
A current challenge, however, is that the blood donation from the recovered patients must be precisely timed in order to maximize the number of antibodies a future patient receives. Doctors in China say that obtaining the necessary blood samples at the right time is one of the major barriers to applying the treatment on a larger scale.
"It's difficult to get the donations," said Dr. Yuan Shi of Chongqing Medical University. "When patients have recovered from the disease, we would like to collect their blood two to four weeks afterwards. We try our best to call back the patients, but it's sometimes difficult to get them to come back within that time period."
Because of such hurdles, Japan's largest drugmaker, Takeda Pharmaceuticals, is now working to turn neutralizing antibodies from recovered COVID-19 patients into a standardized drug product. They hope to launch a clinical trial for this in the next few months.
In the U.S., Casadevall hopes blood transfusions from recovered patients can become clinically available as a therapy within the next four weeks, once regulatory approval has been received. Some of the first people to be treated are likely to be doctors and nurses in hospitals who are most at risk of exposure, to provide a protective boost in their immunity.
"A lot of healthcare workers in the U.S. have already been asked to quarantine, and you can imagine what effect that's going to have on the healthcare system," he said. "It can't take large numbers of people staying home; there's not the capacity."
But not all medical experts are convinced it's the way to go, especially when it comes to the most severe cases of COVID-19. "There's no knowing whether that treatment would be useful or not," warned Dr. Andrew Freedman, head of Cardiff University's School of Medicine in the U.K.
"There are going to be better things available in a few months, but we are facing, 'What do you do now?'"
However, Casadevall says that the treatment is not envisioned as a panacea to treating coronavirus, but simply a temporary measure which could give doctors some options until stronger options such as vaccines or new drugs are available.
"This is a stopgap option," he said. "There are going to be better things available in a few months, but we are facing, 'What do you do now?' The only thing we can offer severely ill people at the moment is respiratory support and oxygen, and we don't have anything to prevent those exposed from going on and getting ill."
Social distancing is a critical part of the strategy to defeat the novel coronavirus.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.