Podcast: Should Scientific Controversies Be Silenced?
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.

The recent Joe Rogan/Spotify controversy prompts the consideration of tough questions about expertise, trust, gatekeepers, and dissent.
The "Making Sense of Science" podcast features interviews with leading medical and scientific experts about the latest developments and the big ethical and societal questions they raise. This monthly podcast is hosted by journalist Kira Peikoff, founding editor of the award-winning science outlet Leaps.org.
The recent Joe Rogan/Spotify backlash over the misinformation presented in his recent episode on the Covid-19 vaccines raises some difficult and important bioethical questions for society: How can people know which experts to trust? What should big tech gatekeepers do about false claims promoted on their platforms? How should the scientific establishment respond to heterodox viewpoints from experts who disagree with the consensus? When is silencing of dissent merited, and when is it problematic? Journalist Kira Peikoff asks infectious disease physician and pandemic scholar Dr. Amesh Adalja to weigh in.

Dr. Amesh Adalja, Senior Scholar, Johns Hopkins Center for Health Security and an infectious disease physician
Listen to the Episode
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
Podcast: The Friday Five - your health research roundup
The Friday Five is a new series in which Leaps.org covers five breakthroughs in research over the previous week that you may have missed.
The Friday Five is a new podcast series in which Leaps.org covers five breakthroughs in research over the previous week that you may have missed. There are plenty of controversies and ethical issues in science – and we get into many of them in our online magazine – but there’s also plenty to be excited about, and this news roundup is focused on inspiring scientific work to give you some momentum headed into the weekend.
Covered in this week's Friday Five:
- Puffer fish chemical for treating chronic pain
- Sleep study on the health benefits of waking up multiples times per night
- Best exercise regimens for reducing the risk of mortality aka living longer
- AI breakthrough in mapping protein structures with DeepMind
- Ultrasound stickers to see inside your body
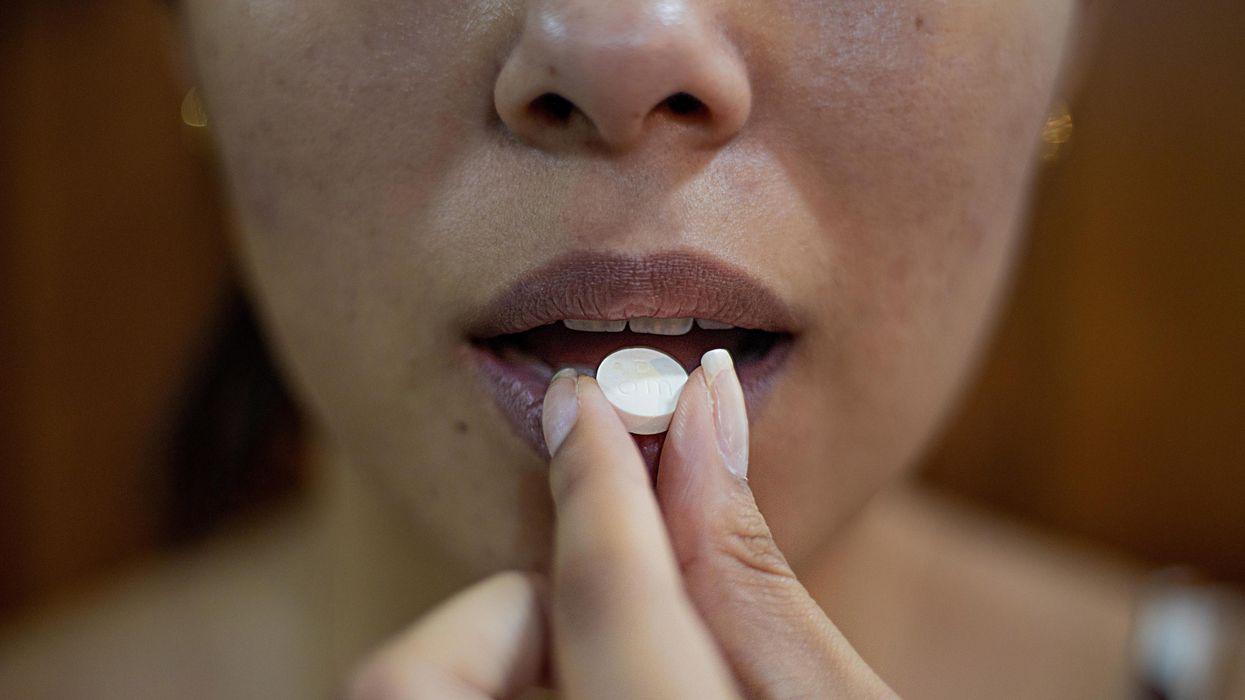
CandyCodes could provide sweet justice against fake pills
A bioengineer at the University of California, Riverside, may have found a way to prevent counterfeit medications: pill coatings inspired by the sprinkles on baked goods and candies.
When we swallow a pill, we hope it will work without side effects. Few of us know to worry about a growing issue facing the pharmaceutical industry: counterfeit medications. These pills, patches, and other medical products might look just like the real thing. But they’re often stuffed with fillers that dilute the medication’s potency or they’re simply substituted for lookalikes that contain none of the prescribed medication at all.
Now, bioengineer William Grover at the University of California, Riverside, may have a solution. Inspired by the tiny, multi-colored sprinkles called nonpareils that decorate baked goods and candies, Grover created CandyCodes pill coatings to prevent counterfeits.
The idea was borne out of pandemic boredom. Confined to his home, Grover was struck by the patterns of nonpareils he saw on candies, and found himself counting the number of little balls on each one. “It’s random, how they’re applied,” he says. “I wondered if it ever repeats itself or if each of these candies is unique in the entire world.” He suspected the latter, and some quick math proved his hypothesis: Given dozens of nonpareils per candy in a handful of different colors, it’s highly unlikely that the sprinklings on any two candies would be identical.
He quickly realized his finding could have practical applications: pills or capsules could be coated with similar “sprinkles,” with the manufacturer photographing each pill or capsule before selling its products. Consumers looking to weed out fakes could potentially take a photo with their cell phones and go online to compare images of their own pills to the manufacturer’s database, with the help of an algorithm that would determine their authenticity. Or, a computer could generate another type of unique identifier, such as a text-based code, tracking to the color and location of the sprinkles. This would allow for a speedier validation than a photo-based comparison, Grover says. “It could be done very quickly, in a fraction of a second.”
Researchers and manufacturers have already developed some anti-counterfeit tools, including built-in identifiers like edible papers with scannable QR codes. But such methods, while functional, can be costly to implement, Grover says.
It wouldn’t be paranoid to take such precautions. Counterfeits are a growing problem, according to Young Kim, a biomedical engineer at Purdue University who was not involved in the CandyCodes study. “There are approximately 40,000 online pharmacies that one can access via the Internet,” he says. “Only three to four percent of them are operated legally.” Purchases from online pharmacies rose dramatically during the pandemic, and Kim expects a boom in counterfeit medical products alongside it.
The FDA warns that U.S. consumers can be exposed to counterfeits through online purchases, in particular. The problem is magnified in low- to middle-income nations, where one in 10 medical products are counterfeit, according to a World Health Organization estimate. Cost doesn’t seem to be a factor, either; antimalarials and antibiotics are most often reported as counterfeits or fakes, and generic medications are swapped as often as brand-name drugs, according to the same WHO report.
Counterfeits weren’t tracked globally until 2013; since then, there have been 1,500 reports to the WHO, with actual incidences of counterfeiting likely much higher. Fake medicines have been estimated to result in costs of $200 billion each year, and are blamed for more than 72,000 pneumonia- and 116,000 malaria-related deaths.
Researchers and manufacturers have already developed some anti-counterfeit tools, including built-in identifiers like edible papers with scannable QR codes or barcodes that are stamped onto or otherwise incorporated into pills and other medical products. But such methods, while functional, can be costly to implement, Grover says.

CandyCodes could provide unique identifiers for at least 41 million pills for every person on the planet.
William Grover
“Putting universal codes on each pill and each dosage is attractive,” he says. “The challenge is, how can we do it in a way that requires as little modification to the existing manufacturing process as possible? That's where I hope CandyCodes have an edge. It's not zero modification, but I hope it is as minor a modification of the manufacturing process as possible.”
Kim calls the concept “a clever idea to introduce entropy for high-level security” even if it may not be as close to market as other emerging technologies, including some edible watermarks he’s helped develop. He points out that CandyCodes still needs to be tested for reproducibility and readability.
The possibilities are already intriguing, though. Grover’s recent research, published in Scientific Reports, predicts that unique codes could be used for at least 41 million pills for every person on the planet.
Sadly, CandyCodes’ multicolored bits probably won’t taste like candy. They must be made of non-caloric ingredients to meet the international regulatory standards that govern food dyes and colorants. But Grover hopes CandyCodes represent a simple, accessible solution to a heart-wrenching issue. “This feels like trying to track down and go after bad guys,” he says. “Someone who would pass off a medicine intended for a child or a sick person and pass it off as something effective, I can't imagine anything much more evil than that. It's fun and, and a little fulfilling to try to develop technologies that chip away at that.”