Saliva Testing Offers Easier and Earlier Detection of COVID-19

Dr. Andrew Brooks of RUCDR Infinite Biologics holds up a saliva sample.
The patient tilts back her head and winces as the long swab stick pushes six inches up her nose. The tip twirls around uncomfortably before it's withdrawn.
"Our saliva test can detect the virus in asymptomatic and pre-symptomatic cases."
A gloved and gowned healthcare worker wearing a face shield and mask tells the patient that she will learn whether she is positive for COVID-19 as soon as the lab can process her test.
This is the typical unpleasant scenario for getting a coronavirus test. But times are rapidly changing: Today, for the first time, the U.S. Food and Drug Administration cleared one company to sell saliva collection kits for individuals to use at home.
Scientists at the startup venture, RUCDR Infinite Biologics at Rutgers University in New Jersey, say that saliva testing offers an easier, more useful alternative to the standard nasal swab.
"Our saliva test can detect the virus in asymptomatic and pre-symptomatic cases," said Dr. Andrew Brooks, chief operating officer at RUCDR.
Another venture, Darwin BioSciences in Colorado, has separately developed an innovative method of testing saliva for the coronavirus that causes COVID-19.
Saliva testing can allow earlier detection to identify people who may not know they are contagious, say scientists at both companies. In addition, because patients spit into a tube or cup, saliva testing is safer for healthcare workers than taking swabs. This frees up scarce personal protective equipment (PPE) for use elsewhere. Nasal swabs themselves have been in scarce supply.
Saliva testing, if it becomes widespread, potentially could mean opening society sooner. The more ubiquitous testing becomes across the population, experts say, the more feasible it becomes for public health officials to trace and isolate contacts, especially of asymptomatic cases. Testing early and often will be essential to containing emerging hot spots before a vast outbreak can take root.
Darwin Biosceiences is preparing to seek an FDA Emergency Use Authorization (EUA) this month for its patented "CoVScreen" testing system, which potentially could be available to labs nationally by mid-summer.
Meanwhile, Infinite Biologics will now begin selling kits to consumers for home collection, upon order by a physician. The FDA said that the company's saliva test was as accurate as the nasal swab method used by health care professionals. An FDA summary documenting the company's data reported: "There was 100% positive and negative agreement between the results obtained from testing of saliva and those obtained from nasopharyngeal and oropharyngeal swabs."
The greatest scientific advantage, said Dr. Brooks, is that nasal and oral swabs only collect the surface area where the swab goes, which may not be the place with most viral load. In contrast, the virus occurs throughout a saliva sample, so the test is more trustworthy.
The lab at Rutgers can process 20,000 tests a day, with a 48-hour turnaround. They have 75,000 tests ready to ship now.
The Leap: Detecting Sickness Before You Feel It
"We wanted to create a device that could detect infections before symptoms appeared," explained Nicholas Meyerson, co-founder and CEO of Darwin.
For more than 300 years, he said, "the thermometer was the gold standard for detecting disease because we thought the first sign of illness was a fever. This COVID-19 pandemic has proven that not all pathogens cause a fever. You can be highly contagious without knowing it."
"The question is whether we can scale up fast enough to meet the need. I believe saliva testing can help."
Therefore, Meyerson and co-founder Sara Sawyer from the University of Colorado began to identify RNA biomarkers that can sense when a pathogen first enters a molecule and "sets off alarms." They focused on the nucleic acids concentrated in saliva as the best and easiest place to collect samples for testing.
"The isothermal reaction in saliva takes place at body or room temperature," he said, "so there's no need for complicated testing machinery. The chemical reaction can be read out on a paper strip, like a pregnancy test -- two stripes if you're sick, and one stripe if you're okay."
Before the pandemic, limited but successful human trials were already underway at CU in Boulder and at the CU Anschutz Medical Campus east of Denver. "This was our proof of concept," he said.
Darwin was founded in March and has secured enough venture capital to concentrate protype development on detecting the virus causing COVID-19. So far, said Meyerson, "Everything works."
A small double-blind test of 30 samples at CU produced 100 percent accuracy. "I'm not sure if that will hold true as we go into clinical trials," he said, "but I'm confident we will satisfy all the requirements for at least 95 percent clinical validation."
The specific "CoVStick" test strips will roll out soon, he said: "We hope before the second wave of the pandemic hits."
The broader saliva test-strip product from Darwin, "SickStick," is still one to two years away from deployment by the military and introduction into the consumer drugstore market for home use, said Meyerson. It will affordably and quickly detect a range of viral and bacterial infections.

An illustration of the "CoVStick."
(Darwin Biosciences)
A Potential Game Changer
Society needs widespread testing daily, said George Church, founding core faculty of the Wyss Institute for Biologically Inspired Engineering at Harvard University. Speaking at an online SynBioBeta webinar in April, he urged developing stockpiles of testing kits for home use.
As for any potential of false positives, Church said a much bigger risk is not having enough tests.
"Saliva testing is going to speed up the timeline for opening society a lot," said Meyerson. "People need to self-collect samples at home. A lot more people are going to be willing to spit into a tube than to push a swab six inches up their own nose."
Brooks, of Rutgers, addressed the big picture. "It's critical that we open society as soon as possible to minimize the economic impact of the pandemic. Testing is the surest and safest path. The question is whether we can scale up fast enough to meet the need. I believe saliva testing can help."
Is a Successful HIV Vaccine Finally on the Horizon?
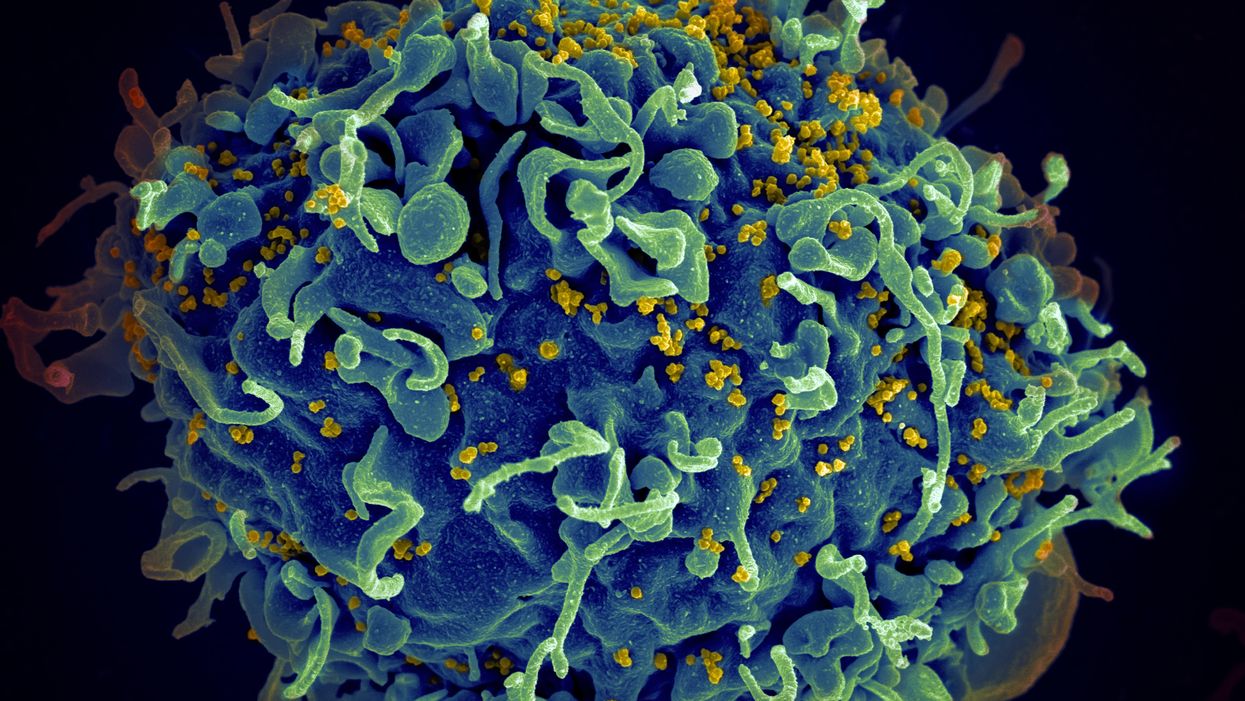
The HIV virus (yellow) infecting a human cell.
Few vaccines have been as complicated—and filled with false starts and crushed hopes—as the development of an HIV vaccine.
While antivirals help HIV-positive patients live longer and reduce viral transmission to virtually nil, these medications must be taken for life, and preventative medications like pre-exposure prophylaxis, known as PrEP, need to be taken every day to be effective. Vaccines, even if they need boosters, would make prevention much easier.
In August, Moderna began human trials for two HIV vaccine candidates based on messenger RNA.
As they have with the Covid-19 pandemic, mRNA vaccines could change the game. The technology could be applied for gene editing therapy, cancer, other infectious diseases—even a universal influenza vaccine.
In the past, three other mRNA vaccines completed phase-2 trials without success. But the easily customizable platforms mean the vaccines can be tweaked better to target HIV as researchers learn more.
Ever since HIV was discovered as the virus causing AIDS, researchers have been searching for a vaccine. But the decades-long journey has so far been fruitless; while some vaccine candidates showed promise in early trials, none of them have worked well among later-stage clinical trials.
There are two main reasons for this: HIV evolves incredibly quickly, and the structure of the virus makes it very difficult to neutralize with antibodies.
"We in HIV medicine have been desperate to find a vaccine that has effectiveness, but this goal has been elusive so far."
"You know the panic that goes on when a new coronavirus variant surfaces?" asked John Moore, professor of microbiology and immunology at Weill Cornell Medicine who has researched HIV vaccines for 25 years. "With HIV, that kind of variation [happens] pretty much every day in everybody who's infected. It's just orders of magnitude more variable a virus."
Vaccines like these usually work by imitating the outer layer of a virus to teach cells how to recognize and fight off the real thing off before it enters the cell. "If you can prevent landing, you can essentially keep the virus out of the cell," said Larry Corey, the former president and director of the Fred Hutchinson Cancer Research Center who helped run a recent trial of a Johnson & Johnson HIV vaccine candidate, which failed its first efficacy trial.
Like the coronavirus, HIV also has a spike protein with a receptor-binding domain—what Moore calls "the notorious RBD"—that could be neutralized with antibodies. But while that target sticks out like a sore thumb in a virus like SARS-CoV-2, in HIV it's buried under a dense shield. That's not the only target for neutralizing the virus, but all of the targets evolve rapidly and are difficult to reach.
"We understand these targets. We know where they are. But it's still proving incredibly difficult to raise antibodies against them by vaccination," Moore said.
In fact, mRNA vaccines for HIV have been under development for years. The Covid vaccines were built on decades of that research. But it's not as simple as building on this momentum, because of how much more complicated HIV is than SARS-CoV-2, researchers said.
"They haven't succeeded because they were not designed appropriately and haven't been able to induce what is necessary for them to induce," Moore said. "The mRNA technology will enable you to produce a lot of antibodies to the HIV envelope, but if they're the wrong antibodies that doesn't solve the problem."
Part of the problem is that the HIV vaccines have to perform better than our own immune systems. Many vaccines are created by imitating how our bodies overcome an infection, but that doesn't happen with HIV. Once you have the virus, you can't fight it off on your own.
"The human immune system actually does not know how to innately cure HIV," Corey said. "We needed to improve upon the human immune system to make it quicker… with Covid. But we have to actually be better than the human immune system" with HIV.
But in the past few years, there have been impressive leaps in understanding how an HIV vaccine might work. Scientists have known for decades that neutralizing antibodies are key for a vaccine. But in 2010 or so, they were able to mimic the HIV spike and understand how antibodies need to disable the virus. "It helps us understand the nature of the problem, but doesn't instantly solve the problem," Moore said. "Without neutralizing antibodies, you don't have a chance."
Because the vaccines need to induce broadly neutralizing antibodies, and because it's very difficult to neutralize the highly variable HIV, any vaccine will likely be a series of shots that teach the immune system to be on the lookout for a variety of potential attacks.
"Each dose is going to have to have a different purpose," Corey said. "And we hope by the end of the third or fourth dose, we will achieve the level of neutralization that we want."
That's not ideal, because each individual component has to be made and tested—and four shots make the vaccine harder to administer.
"You wouldn't even be going down that route, if there was a better alternative," Moore said. "But there isn't a better alternative."
The mRNA platform is exciting because it is easily customizable, which is especially important in fighting against a shapeshifting, complicated virus. And the mRNA platform has shown itself, in the Covid pandemic, to be safe and quick to make. Effective Covid vaccines were comparatively easy to develop, since the coronavirus is easier to battle than HIV. But companies like Moderna are capitalizing on their success to launch other mRNA therapeutics and vaccines, including the HIV trial.
"You can make the vaccine in two months, three months, in a research lab, and not a year—and the cost of that is really less," Corey said. "It gives us a chance to try many more options, if we've got a good response."
In a trial on macaque monkeys, the Moderna vaccine reduced the chances of infection by 85 percent. "The mRNA platform represents a very promising approach for the development of an HIV vaccine in the future," said Dr. Peng Zhang, who is helping lead the trial at the National Institute of Allergy and Infectious Diseases.
Moderna's trial in humans represents "a very exciting possibility for the prevention of HIV infection," Dr. Monica Gandhi, director of the UCSF-Gladstone Center for AIDS Research, said in an email. "We in HIV medicine have been desperate to find a vaccine that has effectiveness, but this goal has been elusive so far."
If a successful HIV vaccine is developed, the series of shots could include an mRNA shot that primes the immune system, followed by protein subunits that generate the necessary antibodies, Moore said.
"I think it's the only thing that's worth doing," he said. "Without something complicated like that, you have no chance of inducing broadly neutralizing antibodies."
"I can't guarantee you that's going to work," Moore added. "It may completely fail. But at least it's got some science behind it."
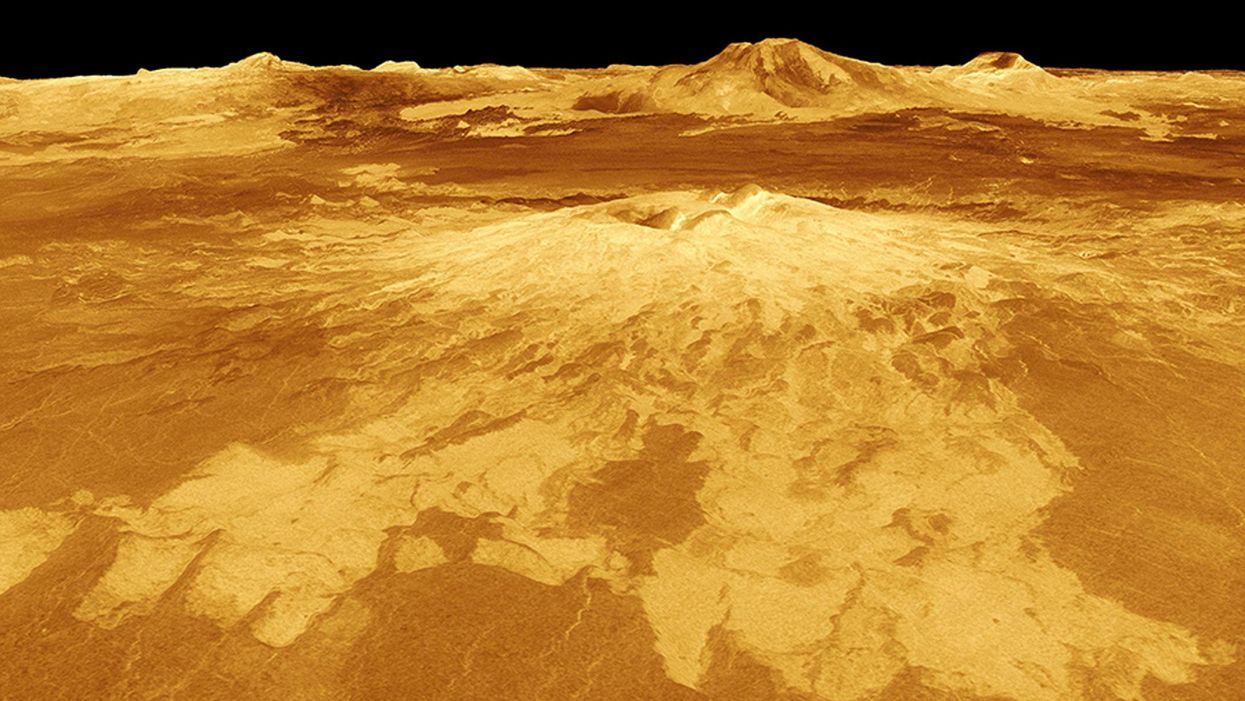
New Podcast: The Lead Scientist for the NASA Mission to Venus
The volcano Sapas Mons is displayed in the center of this computer-generated three-dimensional perspective view of the surface of Venus.
The "Making Sense of Science" podcast features interviews with leading medical and scientific experts about the latest developments and the big ethical and societal questions they raise. This monthly podcast is hosted by journalist Kira Peikoff, founding editor of the award-winning science outlet Leaps.org.
This month, our guest is JPL's Dr. Suzanne Smrekar, who will be pushing the boundaries of knowledge about the planet Venus during the upcoming VERITAS mission set to launch in 2028. Why did Earth's twin planet develop so differently than our own? Could Venus ever have hosted life? What is the bigger purpose for humanity in studying the solar system -- is it purely scientific, or is it also a matter of art and philosophy? Hear Dr. Smrekar discuss all this and more on the latest episode.
Watch the 30-Second Trailer:
Listen to the Episode:
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.