Saliva Testing Offers Easier and Earlier Detection of COVID-19

Dr. Andrew Brooks of RUCDR Infinite Biologics holds up a saliva sample.
The patient tilts back her head and winces as the long swab stick pushes six inches up her nose. The tip twirls around uncomfortably before it's withdrawn.
"Our saliva test can detect the virus in asymptomatic and pre-symptomatic cases."
A gloved and gowned healthcare worker wearing a face shield and mask tells the patient that she will learn whether she is positive for COVID-19 as soon as the lab can process her test.
This is the typical unpleasant scenario for getting a coronavirus test. But times are rapidly changing: Today, for the first time, the U.S. Food and Drug Administration cleared one company to sell saliva collection kits for individuals to use at home.
Scientists at the startup venture, RUCDR Infinite Biologics at Rutgers University in New Jersey, say that saliva testing offers an easier, more useful alternative to the standard nasal swab.
"Our saliva test can detect the virus in asymptomatic and pre-symptomatic cases," said Dr. Andrew Brooks, chief operating officer at RUCDR.
Another venture, Darwin BioSciences in Colorado, has separately developed an innovative method of testing saliva for the coronavirus that causes COVID-19.
Saliva testing can allow earlier detection to identify people who may not know they are contagious, say scientists at both companies. In addition, because patients spit into a tube or cup, saliva testing is safer for healthcare workers than taking swabs. This frees up scarce personal protective equipment (PPE) for use elsewhere. Nasal swabs themselves have been in scarce supply.
Saliva testing, if it becomes widespread, potentially could mean opening society sooner. The more ubiquitous testing becomes across the population, experts say, the more feasible it becomes for public health officials to trace and isolate contacts, especially of asymptomatic cases. Testing early and often will be essential to containing emerging hot spots before a vast outbreak can take root.
Darwin Biosceiences is preparing to seek an FDA Emergency Use Authorization (EUA) this month for its patented "CoVScreen" testing system, which potentially could be available to labs nationally by mid-summer.
Meanwhile, Infinite Biologics will now begin selling kits to consumers for home collection, upon order by a physician. The FDA said that the company's saliva test was as accurate as the nasal swab method used by health care professionals. An FDA summary documenting the company's data reported: "There was 100% positive and negative agreement between the results obtained from testing of saliva and those obtained from nasopharyngeal and oropharyngeal swabs."
The greatest scientific advantage, said Dr. Brooks, is that nasal and oral swabs only collect the surface area where the swab goes, which may not be the place with most viral load. In contrast, the virus occurs throughout a saliva sample, so the test is more trustworthy.
The lab at Rutgers can process 20,000 tests a day, with a 48-hour turnaround. They have 75,000 tests ready to ship now.
The Leap: Detecting Sickness Before You Feel It
"We wanted to create a device that could detect infections before symptoms appeared," explained Nicholas Meyerson, co-founder and CEO of Darwin.
For more than 300 years, he said, "the thermometer was the gold standard for detecting disease because we thought the first sign of illness was a fever. This COVID-19 pandemic has proven that not all pathogens cause a fever. You can be highly contagious without knowing it."
"The question is whether we can scale up fast enough to meet the need. I believe saliva testing can help."
Therefore, Meyerson and co-founder Sara Sawyer from the University of Colorado began to identify RNA biomarkers that can sense when a pathogen first enters a molecule and "sets off alarms." They focused on the nucleic acids concentrated in saliva as the best and easiest place to collect samples for testing.
"The isothermal reaction in saliva takes place at body or room temperature," he said, "so there's no need for complicated testing machinery. The chemical reaction can be read out on a paper strip, like a pregnancy test -- two stripes if you're sick, and one stripe if you're okay."
Before the pandemic, limited but successful human trials were already underway at CU in Boulder and at the CU Anschutz Medical Campus east of Denver. "This was our proof of concept," he said.
Darwin was founded in March and has secured enough venture capital to concentrate protype development on detecting the virus causing COVID-19. So far, said Meyerson, "Everything works."
A small double-blind test of 30 samples at CU produced 100 percent accuracy. "I'm not sure if that will hold true as we go into clinical trials," he said, "but I'm confident we will satisfy all the requirements for at least 95 percent clinical validation."
The specific "CoVStick" test strips will roll out soon, he said: "We hope before the second wave of the pandemic hits."
The broader saliva test-strip product from Darwin, "SickStick," is still one to two years away from deployment by the military and introduction into the consumer drugstore market for home use, said Meyerson. It will affordably and quickly detect a range of viral and bacterial infections.

An illustration of the "CoVStick."
(Darwin Biosciences)
A Potential Game Changer
Society needs widespread testing daily, said George Church, founding core faculty of the Wyss Institute for Biologically Inspired Engineering at Harvard University. Speaking at an online SynBioBeta webinar in April, he urged developing stockpiles of testing kits for home use.
As for any potential of false positives, Church said a much bigger risk is not having enough tests.
"Saliva testing is going to speed up the timeline for opening society a lot," said Meyerson. "People need to self-collect samples at home. A lot more people are going to be willing to spit into a tube than to push a swab six inches up their own nose."
Brooks, of Rutgers, addressed the big picture. "It's critical that we open society as soon as possible to minimize the economic impact of the pandemic. Testing is the surest and safest path. The question is whether we can scale up fast enough to meet the need. I believe saliva testing can help."
Recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen as soon as 18 months from now
Like all those whose kidneys have failed, Scott Burton’s life revolves around dialysis. For nearly two decades, Burton has been hooked up (or, since 2020, has hooked himself up at home) to a dialysis machine that performs the job his kidneys normally would. The process is arduous, time-consuming, and expensive. Except for a brief window before his body rejected a kidney transplant, Burton has depended on machines to take the place of his kidneys since he was 12-years-old. His whole life, the 39-year-old says, revolves around dialysis.
“Whenever I try to plan anything, I also have to plan my dialysis,” says Burton says, who works as a freelance videographer and editor. “It’s a full-time job in itself.”
Many of those on dialysis are in line for a kidney transplant that would allow them to trade thrice-weekly dialysis and strict dietary limits for a lifetime of immunosuppressants. Burton’s previous transplant means that his body will likely reject another donated kidney unless it matches perfectly—something he’s not counting on. It’s why he’s enthusiastic about the development of artificial kidneys, small wearable or implantable devices that would do the job of a healthy kidney while giving users like Burton more flexibility for traveling, working, and more.
Still, the devices aren’t ready for testing in humans—yet. But recent advancements in engineering mean that the first preclinical trials for an artificial kidney could happen as soon as 18 months from now, according to Jonathan Himmelfarb, a nephrologist at the University of Washington.
“It would liberate people with kidney failure,” Himmelfarb says.
An engineering marvel
Compared to the heart or the brain, the kidney doesn’t get as much respect from the medical profession, but its job is far more complex. “It does hundreds of different things,” says UCLA’s Ira Kurtz.
Kurtz would know. He’s worked as a nephrologist for 37 years, devoting his career to helping those with kidney disease. While his colleagues in cardiology and endocrinology have seen major advances in the development of artificial hearts and insulin pumps, little has changed for patients on hemodialysis. The machines remain bulky and require large volumes of a liquid called dialysate to remove toxins from a patient’s blood, along with gallons of purified water. A kidney transplant is the next best thing to someone’s own, functioning organ, but with over 600,000 Americans on dialysis and only about 100,000 kidney transplants each year, most of those in kidney failure are stuck on dialysis.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. Each of the 45 different cell types in the kidney do something different.
Part of the lack of progress in artificial kidney design is the sheer complexity of the kidney’s job. To build an artificial heart, Kurtz says, you basically need to engineer a pump. An artificial pancreas needs to balance blood sugar levels with insulin secretion. While neither of these tasks is simple, they are fairly straightforward. The kidney, on the other hand, does more than get rid of waste products like urea and other toxins. Each of the 45 different cell types in the kidney do something different, helping to regulate electrolytes like sodium, potassium, and phosphorous; maintaining blood pressure and water balance; guiding the body’s hormonal and inflammatory responses; and aiding in the formation of red blood cells.

There's been little progress for patients during Ira Kurtz's 37 years as a nephrologist. Artificial kidneys would change that.
UCLA
Dialysis primarily filters waste, and does so well enough to keep someone alive, but it isn’t a true artificial kidney because it doesn’t perform the kidney’s other jobs, according to Kurtz, such as sensing levels of toxins, wastes, and electrolytes in the blood. Due to the size and water requirements of existing dialysis machines, the equipment isn’t portable. Physicians write a prescription for a certain duration of dialysis and assess how well it’s working with semi-regular blood tests. The process of dialysis itself, however, is conducted blind. Doctors can’t tell how much dialysis a patient needs based on kidney values at the time of treatment, says Meera Harhay, a nephrologist at Drexel University in Philadelphia.
But it’s the impact of dialysis on their day-to-day lives that creates the most problems for patients. Only one-quarter of those on dialysis are able to remain employed (compared to 85% of similar-aged adults), and many report a low quality of life. Having more flexibility in life would make a major different to her patients, Harhay says.
“Almost half their week is taken up by the burden of their treatment. It really eats away at their freedom and their ability to do things that add value to their life,” she says.
Art imitates life
The challenge for artificial kidney designers was how to compress the kidney’s natural functions into a portable, wearable, or implantable device that wouldn’t need constant access to gallons of purified and sterilized water. The other universal challenge they faced was ensuring that any part of the artificial kidney that would come in contact with blood was kept germ-free to prevent infection.
As part of last year’s KidneyX Prize, a partnership between the U.S. Department of Health and Human Services and the American Society of Nephrology, inventors were challenged to create prototypes for artificial kidneys. Himmelfarb’s team at the University of Washington’s Center for Dialysis Innovation won the prize by focusing on miniaturizing existing technologies to create a portable dialysis machine. The backpack sized AKTIV device (Ambulatory Kidney to Increase Vitality) will recycle dialysate in a closed loop system that removes urea from blood and uses light-based chemical reactions to convert the urea to nitrogen and carbon dioxide, which allows the dialysate to be recirculated.
Himmelfarb says that the AKTIV can be used when at home, work, or traveling, which will give users more flexibility and freedom. “If you had a 30-pound device that you could put in the overhead bins when traveling, you could go visit your grandkids,” he says.
Kurtz’s team at UCLA partnered with the U.S. Kidney Research Corporation and Arkansas University to develop a dialysate-free desktop device (about the size of a small printer) as the first phase of a progression that will he hopes will lead to something small and implantable. Part of the reason for the artificial kidney’s size, Kurtz says, is the number of functions his team are cramming into it. Not only will it filter urea from blood, but it will also use electricity to help regulate electrolyte levels in a process called electrodeionization. Kurtz emphasizes that these additional functions are what makes his design a true artificial kidney instead of just a small dialysis machine.
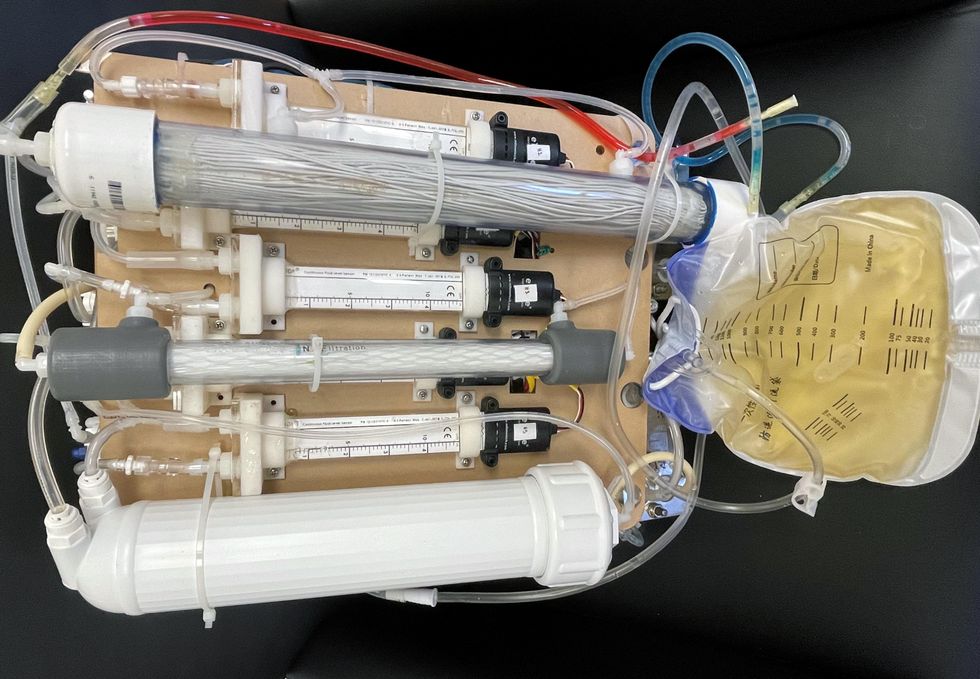
One version of an artificial kidney.
UCLA
“It doesn't have just a static function. It has a bank of sensors that measure chemicals in the blood and feeds that information back to the device,” Kurtz says.
Other startups are getting in on the game. Nephria Bio, a spinout from the South Korean-based EOFlow, is working to develop a wearable dialysis device, akin to an insulin pump, that uses miniature cartridges with nanomaterial filters to clean blood (Harhay is a scientific advisor to Nephria). Ian Welsford, Nephria’s co-founder and CTO, says that the device’s design means that it can also be used to treat acute kidney injuries in resource-limited settings. These potentials have garnered interest and investment in artificial kidneys from the U.S. Department of Defense.
For his part, Burton is most interested in an implantable device, as that would give him the most freedom. Even having a regular outpatient procedure to change batteries or filters would be a minor inconvenience to him.
“Being plugged into a machine, that’s not mimicking life,” he says.
Today’s more than 20,000 mental health apps have a wide range of functionalities and business models. Many of them can be useful for depression.
Even before the pandemic created a need for more telehealth options, depression was a hot area of research for app developers. Given the high prevalence of depression and its connection to suicidality — especially among today’s teenagers and young adults who grew up with mobile devices, use them often, and experience these conditions with alarming frequency — apps for depression could be not only useful but lifesaving.
“For people who are not depressed, but have been depressed in the past, the apps can be helpful for maintaining positive thinking and behaviors,” said Andrea K. Wittenborn, PhD, director of the Couple and Family Therapy Doctoral Program and a professor in human development and family studies at Michigan State University. “For people who are mildly to severely depressed, apps can be a useful complement to working with a mental health professional.”
Health and fitness apps, in general, number in the hundreds of thousands. These are driving a market expected to reach $102.45 billion by next year. The mobile mental health app market is a small part of this but still sizable at $500 million, with revenues generated through user health insurance, employers, and direct payments from individuals.
Apps can provide data that health professionals cannot gather on their own. People’s constant interaction with smartphones and wearable devices yields data on many health conditions for millions of patients in their natural environments and while they go about their usual activities. Compared with the in-office measurements of weight and blood pressure and the brevity of doctor-patient interactions, the thousands of data points gathered unobtrusively over an extended time period provide a far better and more detailed picture of the person and their health.
At their most advanced level, apps for mental health, including depression, passively gather data on how the user touches and interacts with the mobile device through changes in digital biomarkers that relate to depressive symptoms and other conditions.
Building on three decades of research since early “apps” were used for delivering treatment manuals to health professionals, today’s more than 20,000 mental health apps have a wide range of functionalities and business models. Many of these apps can be useful for depression.
Some apps primarily provide a virtual connection to a group of mental health professionals employed or contracted by the app. Others have options for meditation, sleeping or, in the case of industry leaders Calm and Headspace, overall well-being. On the cutting edge are apps that detect changes in a person’s use of mobile devices and their interactions with them.
Apps such as AbleTo, Happify Health, and Woebot Health focus on cognitive behavioral therapy, a type of counseling with proven potential to change a person’s behaviors and feelings. “CBT has been demonstrated in innumerable studies over the last several decades to be effective in the treatment of behavioral health conditions such as depression and anxiety disorders,” said Dr. Reena Pande, chief medical officer at AbleTo. “CBT is intended to be delivered as a structured intervention incorporating key elements, including behavioral activation and adaptive thinking strategies.”
These CBT skills help break the negative self-talk (rumination) common in patients with depression. They are taught and reinforced by some self-guided apps, using either artificial intelligence or programmed interactions with users. Apps can address loneliness and isolation through connections with others, even when a symptomatic person doesn’t feel like leaving the house.
At their most advanced level, apps for mental health, including depression, passively gather data on how the user touches and interacts with the mobile device through changes in “digital biomarkers” that can be associated with onset or worsening of depressive symptoms and other cognitive conditions. In one study, Mindstrong Health gathered a year’s worth of data on how people use their smartphones, such as scrolling through articles, typing and clicking. Mindstrong, whose founders include former leaders of the National Institutes of Health, modeled the timing and order of these actions to make assessments that correlated closely with gold-standard tests of cognitive function.
National organizations of mental health professionals have been following the expanding number of available apps over the years with keen interest. App Advisor is an initiative of the American Psychiatric Association that helps psychiatrists and other mental health professionals navigate the issues raised by mobile health technology. App Advisor does not rate or recommend particular apps but rather provides guidance about why apps should be assessed and how health professionals can do this.
A website that does review mental health apps is One Mind Psyber Guide, an independent nonprofit that partners with several national organizations. One Mind users can select among numerous search terms for the condition and therapeutic approach of interest. Apps are rated on a five-point scale, with reviews written by professionals in the field.
Do mental health apps related to depression have the kind of safety and effectiveness data required for medications and other medical interventions? Not always — and not often. Yet the overall results have shown early promise, Wittenborn noted.
“Studies that have attempted to detect depression from smartphone and wearable sensors [during a single session] have ranged in accuracy from about 86 to 89 percent,” Wittenborn said. “Studies that tried to predict changes in depression over time have been less accurate, with accuracy ranging from 59 to 85 percent.”
The Food and Drug Administration encourages the development of apps and has approved a few of them—mostly ones used by health professionals—but it is generally “hands off,” according to the American Psychiatric Association. The FDA has published a list of examples of software (including programming of apps) that it does not plan to regulate because they pose low risk to the public. First on the list is software that helps patients with diagnosed psychiatric conditions, including depression, maintain their behavioral coping skills by providing a “Skill of the Day” technique or message.
On its App Advisor site, the American Psychiatric Association says mental health apps can be dangerous or cause harm in multiple ways, such as by providing false information, overstating the app’s therapeutic value, selling personal data without clearly notifying users, and collecting data that isn’t relevant to mental health.
Although there is currently reason for caution, patients may eventually come to expect mental health professionals to recommend apps, especially as their rating systems, features and capabilities expand. Through such apps, patients might experience more and higher quality interactions with their mental health professionals. “Apps will continue to be refined and become more effective through future research,” said Wittenborn. “They will become more integrated into practice over time.”