Too much of this ingredient leads to autoimmune diseases, new research shows. Here's how to cut back.

Scientists are looking at how salt affects our cells, and they're finding more reasons to avoid htoo much of it.
For more than a century, doctors have warned that too much salt in your diet can lead to high blood pressure, heart disease and stroke - and many of the reasons for these effects are well known. But recently scientists have been looking deeper, into the cellular level, and they are finding additional reasons to minimize sodium intake; it is bad for immune cells, creating patterns of gene expression and activity seen in a variety of autoimmune diseases such as multiple sclerosis, lupus, rheumatoid arthritis, and type-1 diabetes.
Salt is a major part of the ocean from which life evolved on this planet. We carry that legacy in our blood, which tastes salty. It is an important element for conducting electrical signals along nerves and balancing water and metabolites transported throughout our bodies. We need to consume about 500 milligrams of salt each day to maintain these functions, more with exercise and heavy sweating as that is a major way the body loses salt. The problem is that most Americans eating a modern western diet consume about 3400 milligrams, 1.5 teaspoons per day.
Evidence has been accumulating over the last few years that elevated levels of sodium can be harmful to at least some types of immune cells. The first signal came in monocytes, which are immune cells that travel to various tissues in the body, where some of them turn into macrophages, a subset of white blood cells that can directly kill microorganisms and make chemical signals that bring other types of immune cells into play.
Two years ago, Dominik N. Müller from the Max-Delbrueck-Center in Berlin, Germany and Markus Kleinewietfeld, an immunologist at Hasselt University in Belgium, ran a study where they fed people pizza and then measured their immune cell function. “We saw that in any monocytes, metabolic function was down, even after a single salty meal,” Kleinewietfeld says. It seemed to be the cellular equivalent of the sluggish feeling we get after eating too much. The cells were able to recover but more research is needed to answer questions about what dose of sodium causes impairment, how long the damage lasts, and whether there is a cumulative effect of salt toxicity.
Kleinewietfeld and his colleagues have hypothesized that too much salt could be a significant factor in the increased number of autoimmune diseases and allergies over the last few generations.
The latest series of experiments focused on a type of T cell called T regulatory cells, or Tregs. Most T cells release inflammatory mediators to fight pathogens and, once that job is done, Tregs come along to calm down their hyperactive brethren. Failure to do so can result in continued inflammation and possibly autoimmune diseases.
In the lab, Kleinewietfeld and his large team of international collaborators saw that high levels of sodium had a huge effect on Tregs, upregulating 1250 genes and downregulating an additional 1380 genes so that they looked similar to patterns of gene expression seen in autoimmune diseases.
Digging deeper, they found that sodium affected mitochondria, the tiny organelles inside of cells that produce much of its energy. The sodium was interfering with how the mitochondria use oxygen, which resulted in increased levels of an unstable form of oxygen that can damage cell function. The researchers injected those damaged Tregs into mice and found that they impaired the animals' immune function, allowing the inflammation to continue rather than shutting it down.
That finding dovetailed nicely with a 2019 paper in Nature from Navdeep Chandel's lab at Northwestern University, which showed in mice that inhibiting the mitochondrial use of oxygen reduced the ability of Tregs to regulate other T cells. “Mitochondria were controlling directly the immunosuppressive program, they were this master regulator tuning the right amount of genes to give you proper immunosuppression,” Chandel said. “And if you lose that function, then you get autoimmunity.”
Kleinewietfeld's team studied the Treg cells of humans and found that sodium can similarly decrease mitochondrial use of oxygen and immunosuppressive activity. “I would have never predicted that myself,” Chandel says, but now researchers can look at the mitochondria of patients with autoimmune disease and see if their gene expression also changes under high salt conditions. He sees the link between the patterns of gene expression in Tregs generated by high salt exposure and those patterns seen in autoimmune diseases, but he is cautious about claiming a causal effect.
Kleinewietfeld and his colleagues have hypothesized that too much salt could be a significant factor in the increased number of autoimmune diseases and allergies over the last few generations. He says a high salt diet could also have an indirect effect on immune function through the way it affects the gut microbiome and the molecules made by microbes when they break down food. But the research results are too preliminary to say that for sure, much less parse out the role of salt compared with other possible factors. “It is still an exciting journey to try to understand this field,” he says.
Additionally, it is difficult to say precisely how this research in animals and human cell cultures will translate into a whole human body. Individual differences in genetics can affect how the body absorbs, transports, and gets rid of sodium, such that some people are more sensitive to salt than are others.
So how should people apply these research findings to daily life?
Salt is obvious when we sprinkle it on at the table or eat tasty things like potato chips, but we may be unaware of sodium hidden in packaged foods. That's because salt is an easy and cheap way to boost the flavor of foods. And if we do read the labeled salt content on a package, we focus on the number for a single serving, but then eat more than that.
Last September, the U.S. Food and Drug Administration (FDA) began a process to update labels on the content of food, including what is meant by the word “healthy” and how food manufacturers can use the term. Many in the food industry are resisting those proposed changes.
Chandel cautions against trying to counter the effects of salt by reaching for foods or supplements full of antioxidants, which, in theory, could reduce the harmful effects on mitochondria caused by a heavy hand with the salt shaker.
Until labels are updated, it would be prudent to try to reduce sodium intake by cutting down on packaged foods while making your own food at home, where you know just how much salt has been added. The Mayo Clinic offers guidance on how to become more aware of the sodium in your diet and eat less of it.
Chandel thinks many people will struggle with minimizing salt in their diets. It’s similar to the challenge of eating less sugar, in that the body craves both, and it is difficult to fight that. He cautions against trying to counter the effects of salt by reaching for foods or supplements full of antioxidants, which, in theory, could reduce the harmful effects on mitochondria caused by a heavy hand with the salt shaker. “Dietary antioxidants have failed in just about every clinical trial, yet the public continues to take them,” Chandel says. But he is optimistic that research will lead us to a better understanding of how Tregs function, and uncover new targets for treating autoimmune diseases.
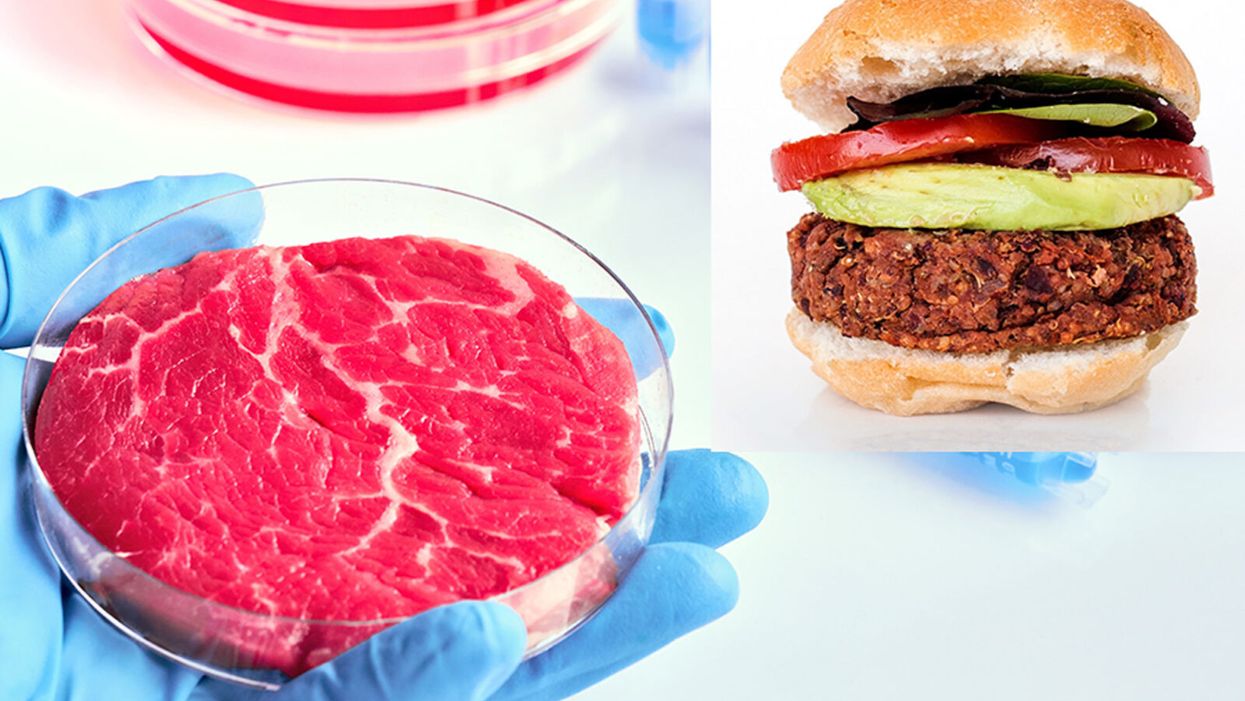
While lab-grown meat is not yet ready to crisis-proof the food supply chain, it offers key benefits that could make it an important solution beyond this pandemic.
The coronavirus pandemic exposed significant weaknesses in the country's food supply chain. Grocery store meat counters were bare. Transportation interruptions influenced supply. Finding beef, poultry, and pork at the store has been, in some places, as challenging as finding toilet paper.
In traditional agriculture models, it takes at least three months to raise chicken, six to nine months for pigs, and 18 months for cattle.
It wasn't a lack of supply -- millions of animals were in the pipeline.
"There's certainly enough food out there, but it can't get anywhere because of the way our system is set up," said Amy Rowat, an associate professor of integrative biology and physiology at UCLA. "Having a more self-contained, self-sufficient way to produce meat could make the supply chain more robust."
Cultured meat could be one way of making the meat supply chain more resilient despite disruptions due to pandemics such as COVID-19. But is the country ready to embrace lab-grown food?
According to a Good Food Institute study, GenZ is almost twice as likely to embrace meat alternatives for reasons related to social and environmental awareness, even prior to the pandemic. That's because this group wants food choices that reflect their values around food justice, equity, and animal welfare.
Largely, the interest in protein alternatives has been plant-based foods. However, factors directly related to COVID-19 may accelerate consumer interest in the scaling up of cell-grown products, according to Liz Specht, the associate director of science and technology at The Good Food Institute. The latter is a nonprofit organization that supports scientists, investors, and entrepreneurs working to develop food alternatives to conventional animal products.
While lab-grown food isn't ready yet to definitively crisis-proof the food supply chain, experts say it offers promise.
Matching Supply and Demand
Companies developing cell-grown meat claim it can take as few as two months to develop a cell into an edible product, according to Anthony Chow, CFA at Agronomics Limited, an investment company focused on meat alternatives. Tissue is taken from an animal and placed in a culture that contains nutrients and proteins the cells need to grow and expand. He cites a Good Food Institute report that claims a 2.5-millimeter sample can grow three and a half tons of meat in 40 days, allowing for exponential growth when needed.
In traditional agriculture models, it takes at least three months to raise chicken, six to nine months for pigs, and 18 months for cattle. To keep enough maturing animals in the pipeline, farms must plan the number of animals to raise months -- even years -- in advance. Lab-grown meat advocates say that because cultured meat supplies can be flexible, it theoretically allows for scaling up or down in significantly less time.
"Supply and demand has drastically changed in some way around the world and cultivated meat processing would be able to adapt much quicker than conventional farming," Chow said.
Scaling Up
Lab-grown meat may provide an eventual solution, but not in the immediate future, said Paul Mozdziak, a professor of physiology at North Carolina State University who researches animal cell culture techniques, transgenic animal production, and muscle biology.
"The challenge is in culture media," he said. "It's going to take some innovation to get the cells to grow at quantities that are going to be similar to what you can get from an animal. These are questions that everybody in the space is working on."
Chow says some of the most advanced cultured meat companies, such as BlueNal, anticipate introducing products to the market midway through next year. However, he thinks COVID-19 has slowed the process. Once introduced, they will be at a premium price, most likely available at restaurants before they hit grocery store shelves.
"I think in five years' time it will be in a different place," he said. "I don't think that this will have relevance for this pandemic, but certainly beyond that."
"Plant-based meats may be perceived as 'alternatives' to meat, whereas lab-grown meat is producing the same meat, just in a much more efficient manner, without the environmental implications."
Of course, all the technological solutions in the world won't solve the problem unless people are open-minded about embracing them. At least for now, a lab-grown burger or bluefin tuna might still be too strange for many people, especially in the U.S.
For instance, a 2019 article published by "Frontiers in Sustainable Food Systems" reflects results from a study of 3,030 consumers showing that 29 percent of U.S. customers, 59 percent of Chinese consumers, and 56 percent of Indian consumers were either 'very' or 'extremely likely' to try cultivated meat.
"Lab-grown meat is genuine meat, at the cellular level, and therefore will match conventional meat with regard to its nutritional content and overall sensory experience. It could be argued that plant-based meat will never be able to achieve this," says Laura Turner, who works with Chow at Agronomics Limited. "Plant-based meats may be perceived as 'alternatives' to meat, whereas lab-grown meat is producing the same meat, just in a much more efficient manner, without the environmental implications."
A Solution Beyond This Pandemic
The coronavirus has done more than raise awareness of the fragility of food supply chains. It has also been a wakeup call for consumers and policy makers that it is time to radically rethink our meat, Specht says. Those factors have elevated the profile of lab-grown meat.
"I think the economy is getting a little bit more steam and if I was an investor, I would be getting excited about it," adds Mozdziak.
Beyond crises, Mozdziak explains that as affluence continues to increase globally, meat consumption increases exponentially. Yet farm animals can only grow so quickly and traditional farming won't be able to keep up.
"Even Tyson is saying that by 2050, there's not going to be enough capacity in the animal meat space to meet demand," he notes. "If we don't look at some innovative technologies, how are we going to overcome that?"
A COVID-19 moonshot program challenged chemists around the world to submit ideas for how best to design a drug to target the virus.
By mid-March, Alpha Lee was growing restless. A pioneer of AI-driven drug discovery, Lee leads a team of researchers at the University of Cambridge, but his lab had been closed amidst the government-initiated lockdowns spreading inexorably across Europe.
If the Moonshot proves successful, they hope it could serve as a future benchmark for finding new medicines for chronic diseases.
Having spoken to his collaborators across the globe – many of whom were seeing their own experiments and research projects postponed indefinitely due to the pandemic – he noticed a similar sense of frustration and helplessness in the face of COVID-19.
While there was talk of finding a novel treatment for the virus, Lee was well aware the process was likely to be long and laborious. Traditional methods of drug discovery risked suffering the same fate as the efforts to find a cure for SARS in the early 2000, which took years and were ultimately abandoned long before a drug ever reached the market.
To avoid such an outcome, Lee was convinced that global collaboration was required. Together with a collection of scientists in the UK, US and Israel, he launched the 'COVID Moonshot' – a project which encouraged chemists worldwide to share their ideas for potential drug designs. If the Moonshot proves successful, they hope it could serve as a future benchmark for finding new medicines for chronic diseases.
Solving a Complex Jigsaw
In February, ShanghaiTech University published the first detailed snapshots of the SARS-CoV-2 coronavirus's proteins using a technique called X-ray crystallography. In particular, they revealed a high-resolution profile of the virus's main protease – the part of its structure that enables it to replicate inside a host – and the main drug target. The images were tantalizing.
"We could see all the tiny pieces sitting in the structure like pieces of a jigsaw," said Lee. "All we needed was for someone to come up with the best idea of joining these pieces together with a drug. Then you'd be left with a strong molecule which sits in the protease, and stops it from working, killing the virus in the process."
Normally, ideas for how best to design such a drug would be kept as carefully guarded secrets within individual labs and companies due to their potential value. But as a result, the steady process of trial and error to reach an optimum design can take years to come to fruition.
However, given the scale of the global emergency, Lee felt that the scientific community would be open to collective brainstorming on a mass scale. "Big Pharma usually wouldn't necessarily do this, but time is of the essence here," he said. "It was a case of, 'Let's just rethink every drug discovery stage to see -- ok, how can we go as fast as we can?'"
On March 13, he launched the COVID moonshot, calling for chemists around the globe to come up with the most creative ideas they could think of, on their laptops at home. No design was too weird or wacky to be considered, and crucially nothing would be patented. The entire project would be done on a not-for-profit basis, meaning that any drug that makes it to market will have been created simply for the good of humanity.
It caught fire: Within just two weeks, more than 2,300 potential drug designs had been submitted. By the middle of July, over 10,000 had been received from scientists around the globe.
The Road Toward Clinical Trials
With so many designs to choose from, the team has been attempting to whittle them down to a shortlist of the most promising. Computational drug discovery experts at Diamond and the Weizmann Institute of Science in Rehovot, Israel, have enabled the Moonshot team to develop algorithms for predicting how quick and easy each design would be to make, and to predict how well each proposed drug might bind to the virus in real life.
The latter is an approach known as computational covalent docking and has previously been used in cancer research. "This was becoming more popular even before COVID-19, with several covalent drugs approved by the FDA in recent years," said Nir London, professor of organic chemistry at the Weizmann Institute, and one of the Moonshot team members. "However, all of these were for oncology. A covalent drug against SARS-CoV-2 will certainly highlight covalent drug-discovery as a viable option."
Through this approach, the team have selected 850 compounds to date, which they have manufactured and tested in various preclinical trials already. Fifty of these compounds - which appear to be especially promising when it comes to killing the virus in a test tube – are now being optimized further.
Lee is hoping that at least one of these potential drugs will be shown to be effective in curing animals of COVID-19 within the next six months, a step that would allow the Moonshot team to reach out to potential pharmaceutical partners to test their compounds in humans.
Future Implications
If the project does succeed, some believe it could open the door to scientific crowdsourcing as a future means of generating novel medicine ideas for other diseases. Frank von Delft, professor of protein science and structural biology at the University of Oxford's Nuffield Department of Medicine, described it as a new form of 'citizen science.'
"There's a vast resource of expertise and imagination that is simply dying to be tapped into," he said.
Others are slightly more skeptical, pointing out that the uniqueness of the current crisis has meant that many scientists were willing to contribute ideas without expecting any future compensation in return. This meant that it was easy to circumvent the traditional hurdles that prevent large-scale global collaborations from happening – namely how to decide who will profit from the final product and who will hold the intellectual property (IP) rights.
"I think it is too early to judge if this is a viable model for future drug discovery," says London. "I am not sure that without the existential threat we would have seen so many contributions, and so many people and institutions willing to waive compensation and future royalties. Many scientists found themselves at home, frustrated that they don't have a way to contribute to the fight against COVID-19, and this project gave them an opportunity. Plus many can get behind the fact that this project has no associated IP and no one will get rich off of this effort. This breaks down a lot of the typical barriers and red-tape for wider collaboration."
"If a drug would sprout from one of these crowdsourced ideas, it would serve as a very powerful argument to consider this mode of drug discovery further in the future."
However the Moonshot team believes that if they can succeed, it will at the very least send a strong statement to policy makers and the scientific community that greater efforts should be made to make such large-scale collaborations more feasible.
"All across the scientific world, we've seen unprecedented adoption of open-science, collaboration and collegiality during this crisis, perhaps recognizing that only a coordinated global effort could address this global challenge," says London. "If a drug would sprout from one of these crowdsourced ideas, it would serve as a very powerful argument to consider this mode of drug discovery further in the future."
[An earlier version of this article was published on June 8th, 2020 as part of a standalone magazine called GOOD10: The Pandemic Issue. Produced as a partnership among LeapsMag, The Aspen Institute, and GOOD, the magazine is available for free online.]