Too much of this ingredient leads to autoimmune diseases, new research shows. Here's how to cut back.

Scientists are looking at how salt affects our cells, and they're finding more reasons to avoid htoo much of it.
For more than a century, doctors have warned that too much salt in your diet can lead to high blood pressure, heart disease and stroke - and many of the reasons for these effects are well known. But recently scientists have been looking deeper, into the cellular level, and they are finding additional reasons to minimize sodium intake; it is bad for immune cells, creating patterns of gene expression and activity seen in a variety of autoimmune diseases such as multiple sclerosis, lupus, rheumatoid arthritis, and type-1 diabetes.
Salt is a major part of the ocean from which life evolved on this planet. We carry that legacy in our blood, which tastes salty. It is an important element for conducting electrical signals along nerves and balancing water and metabolites transported throughout our bodies. We need to consume about 500 milligrams of salt each day to maintain these functions, more with exercise and heavy sweating as that is a major way the body loses salt. The problem is that most Americans eating a modern western diet consume about 3400 milligrams, 1.5 teaspoons per day.
Evidence has been accumulating over the last few years that elevated levels of sodium can be harmful to at least some types of immune cells. The first signal came in monocytes, which are immune cells that travel to various tissues in the body, where some of them turn into macrophages, a subset of white blood cells that can directly kill microorganisms and make chemical signals that bring other types of immune cells into play.
Two years ago, Dominik N. Müller from the Max-Delbrueck-Center in Berlin, Germany and Markus Kleinewietfeld, an immunologist at Hasselt University in Belgium, ran a study where they fed people pizza and then measured their immune cell function. “We saw that in any monocytes, metabolic function was down, even after a single salty meal,” Kleinewietfeld says. It seemed to be the cellular equivalent of the sluggish feeling we get after eating too much. The cells were able to recover but more research is needed to answer questions about what dose of sodium causes impairment, how long the damage lasts, and whether there is a cumulative effect of salt toxicity.
Kleinewietfeld and his colleagues have hypothesized that too much salt could be a significant factor in the increased number of autoimmune diseases and allergies over the last few generations.
The latest series of experiments focused on a type of T cell called T regulatory cells, or Tregs. Most T cells release inflammatory mediators to fight pathogens and, once that job is done, Tregs come along to calm down their hyperactive brethren. Failure to do so can result in continued inflammation and possibly autoimmune diseases.
In the lab, Kleinewietfeld and his large team of international collaborators saw that high levels of sodium had a huge effect on Tregs, upregulating 1250 genes and downregulating an additional 1380 genes so that they looked similar to patterns of gene expression seen in autoimmune diseases.
Digging deeper, they found that sodium affected mitochondria, the tiny organelles inside of cells that produce much of its energy. The sodium was interfering with how the mitochondria use oxygen, which resulted in increased levels of an unstable form of oxygen that can damage cell function. The researchers injected those damaged Tregs into mice and found that they impaired the animals' immune function, allowing the inflammation to continue rather than shutting it down.
That finding dovetailed nicely with a 2019 paper in Nature from Navdeep Chandel's lab at Northwestern University, which showed in mice that inhibiting the mitochondrial use of oxygen reduced the ability of Tregs to regulate other T cells. “Mitochondria were controlling directly the immunosuppressive program, they were this master regulator tuning the right amount of genes to give you proper immunosuppression,” Chandel said. “And if you lose that function, then you get autoimmunity.”
Kleinewietfeld's team studied the Treg cells of humans and found that sodium can similarly decrease mitochondrial use of oxygen and immunosuppressive activity. “I would have never predicted that myself,” Chandel says, but now researchers can look at the mitochondria of patients with autoimmune disease and see if their gene expression also changes under high salt conditions. He sees the link between the patterns of gene expression in Tregs generated by high salt exposure and those patterns seen in autoimmune diseases, but he is cautious about claiming a causal effect.
Kleinewietfeld and his colleagues have hypothesized that too much salt could be a significant factor in the increased number of autoimmune diseases and allergies over the last few generations. He says a high salt diet could also have an indirect effect on immune function through the way it affects the gut microbiome and the molecules made by microbes when they break down food. But the research results are too preliminary to say that for sure, much less parse out the role of salt compared with other possible factors. “It is still an exciting journey to try to understand this field,” he says.
Additionally, it is difficult to say precisely how this research in animals and human cell cultures will translate into a whole human body. Individual differences in genetics can affect how the body absorbs, transports, and gets rid of sodium, such that some people are more sensitive to salt than are others.
So how should people apply these research findings to daily life?
Salt is obvious when we sprinkle it on at the table or eat tasty things like potato chips, but we may be unaware of sodium hidden in packaged foods. That's because salt is an easy and cheap way to boost the flavor of foods. And if we do read the labeled salt content on a package, we focus on the number for a single serving, but then eat more than that.
Last September, the U.S. Food and Drug Administration (FDA) began a process to update labels on the content of food, including what is meant by the word “healthy” and how food manufacturers can use the term. Many in the food industry are resisting those proposed changes.
Chandel cautions against trying to counter the effects of salt by reaching for foods or supplements full of antioxidants, which, in theory, could reduce the harmful effects on mitochondria caused by a heavy hand with the salt shaker.
Until labels are updated, it would be prudent to try to reduce sodium intake by cutting down on packaged foods while making your own food at home, where you know just how much salt has been added. The Mayo Clinic offers guidance on how to become more aware of the sodium in your diet and eat less of it.
Chandel thinks many people will struggle with minimizing salt in their diets. It’s similar to the challenge of eating less sugar, in that the body craves both, and it is difficult to fight that. He cautions against trying to counter the effects of salt by reaching for foods or supplements full of antioxidants, which, in theory, could reduce the harmful effects on mitochondria caused by a heavy hand with the salt shaker. “Dietary antioxidants have failed in just about every clinical trial, yet the public continues to take them,” Chandel says. But he is optimistic that research will lead us to a better understanding of how Tregs function, and uncover new targets for treating autoimmune diseases.
As We Wait for a Vaccine, Scientists Work to Scale Up the Best COVID-19 Antibodies to Give New Patients
In this illustration of immune defense, Y-shaped proteins called antibodies attack a virus.
When we get sick, our immune system sends its soldier cells to the battlefield. Called B-cells, they "examine" the foreign particles that shouldn't be in our bloodstream—and start producing the antibodies, the proteins to neutralize the invaders.
To screen the antibodies, scientists have developed a proprietary way to make the effective ones glow – like a literal "lightbulb" moment.
The better these antibodies are at neutralizing the pathogen, the faster we recover.
The antibodies acquired from COVID-19 survivors already showed promise in treating other patients, but because they must be obtained from people, generating a regular supply is not feasible. To close the gap, researchers are trying to identify the B-cells that make the best antibodies—and then farm them in laboratories at scale.
Scientists at Berkley Lights, a biotechnology company in California, have been screening B-cells from recovered patients and testing the antibodies they release for virus-neutralizing abilities. To screen the antibodies, scientists there have developed a proprietary way to make the effective ones glow – like a literal "lightbulb" moment.
So how does it work? First, the individual B-cells are placed into microscopic chambers called nano-pens, where they secrete the antibodies. Once released, the antibodies are flushed over tiny beads that have bits of the viral particles attached to them, along with special molecules that can emit fluorescent light.
"When an antibody binds to the bead, we see a bright light on the bead," explains John Proctor, the company's senior vice president of antibody therapeutics. "So we can identify which cells are making the antibodies."
Then the antibodies are tested for their ability to counteract the coronavirus's spike proteins, which the virus uses to break into our cells. Not all antibodies are equally good at this crucial defense move—some can block only parts of the virus's machinery, while others can neutralize it completely. Proctor and his colleagues are looking for the latter.
Once scientists identify the best performing B-cells, they crack the cells open—or in scientific terms "lyse" them—and extract the genetic instructions for making the antibodies. As it turns out, B-cells aren't very efficient at pumping out massive amounts of antibodies, so scientists insert these genetic instructions into a different, more prolific type of cell.
Named Chinese Hamster Ovary Cells or CHO, these cells are commonly used in the pharmaceutical industry because they can generate therapeutic proteins en masse. Under the right nutrient conditions, which include a lot of sugar, CHO cells can keep making the antibodies at commercial levels. "They are engineered to operate in very large bioreactors," Proctor explains.
While traditional antibody screening can take three months, the Beacon System can do it in less than 24 hours.
Berkeley Lights' technology has already been used to screen the antibodies of recovered patients from Vanderbilt University Medical Center. In another example, a biotech company GenScript ProBio used the platform to screen mice engineered to have human antibodies for the coronavirus.
In addition to its small, lab-on-a-chip size, Berkeley Lights' system allows scientists to greatly speed up the screening process. While traditional antibody screening can take three months, the Beacon System can do it in less than 24 hours. "We only need one B-cell per pen and a couple of beads to see that fluorescent signal," Proctor says. "It is a more advanced way to process and analyze cells, and that level of sensitivity is unique to our technology."
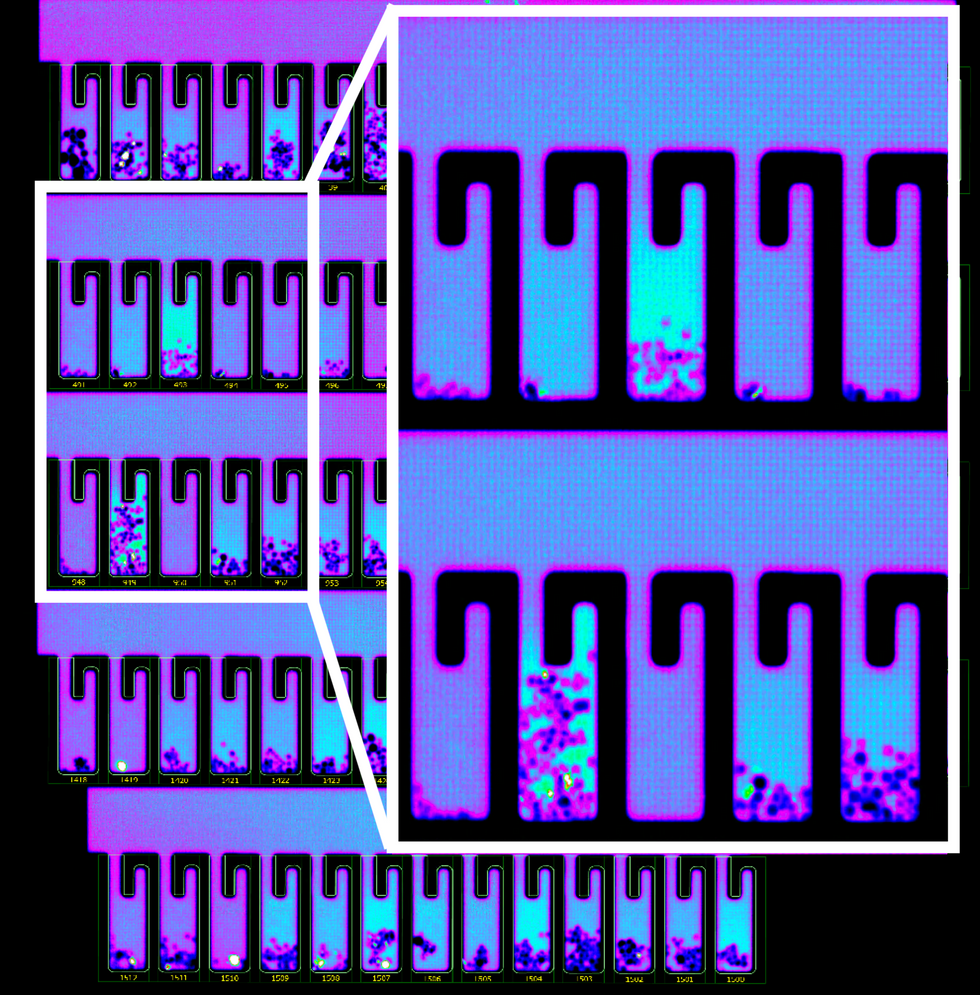
B-cells secreting antibodies inside the Berkeley Lights Beacon System Nano-Pens.
While vaccines are likely to take months to develop and test, antibodies might arrive to the battleground sooner. With the extremely limited treatment options for COVID-19, antibody-based therapeutics can potentially bridge this gap.
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.
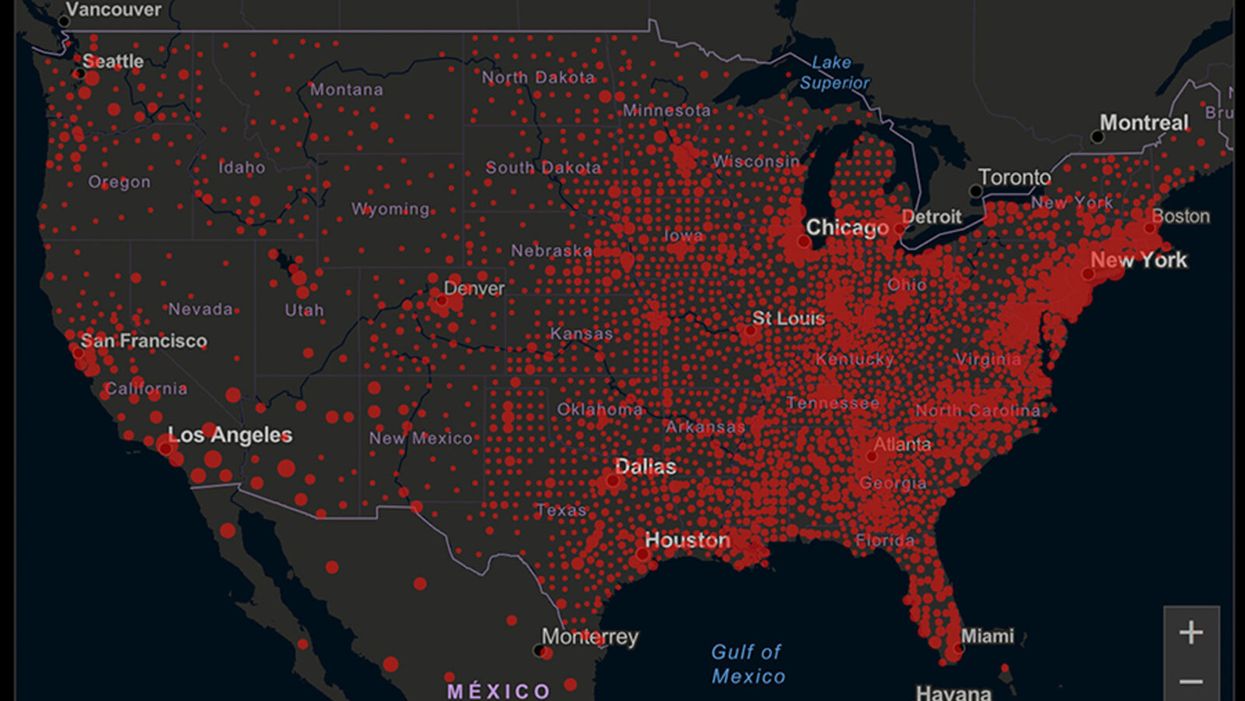
A map of cumulative known cases of COVID-19 in the U.S., as of June 12th, 2020.
Have you felt a bit like an armchair epidemiologist lately? Maybe you've been poring over coronavirus statistics on your county health department's website or on the pages of your local newspaper.
If the percentage of positive tests steadily stays under 8 percent, that's generally a good sign.
You're likely to find numbers and charts but little guidance about how to interpret them, let alone use them to make day-to-day decisions about pandemic safety precautions.
Enter the gurus. We asked several experts to provide guidance for laypeople about how to navigate the numbers. Here's a look at several common COVID-19 statistics along with tips about how to understand them.
Case Counts: Consider the Context
The number of confirmed COVID-19 cases in American counties is widely available. Local and state health departments should provide them online, or you can easily look them up at The New York Times' coronavirus database. However, you need to be cautious about interpreting them.
"Case counts are the obvious numbers to look at. But they're probably the hardest thing to sort out," said Dr. Jeff Martin, an epidemiologist at the University of California at San Francisco.
That's because case counts by themselves aren't a good window into how the coronavirus is affecting your community since they rely on testing. And testing itself varies widely from day to day and community to community.
"The more testing that's done, the more infections you'll pick up," explained Dr. F. Perry Wilson, a physician at Yale University. The numbers can also be thrown off when tests are limited to certain groups of people.
"If the tests are being mostly given to people with a high probability of having been infected -- for example, they have had symptoms or work in a high-risk setting -- then we expect lots of the tests to be positive. But that doesn't tell us what proportion of the general public is likely to have been infected," said Eleanor Murray, an epidemiologist at Boston University.
These Stats Are More Meaningful
According to Dr. Wilson, it's more useful to keep two other statistics in mind: the number of COVID tests that are being performed in your community and the percentage that turn up positive, showing that people have the disease. (These numbers may or may not be available locally. Check the websites of your community's health department and local news media outlets.)
If the number of people being tested is going up, but the percentage of positive tests is going down, Dr. Wilson said, that's a good sign. But if both numbers are going up – the number of people tested and the percentage of positive results – then "that's a sign that there are more infections burning in the community."
It's especially worrisome if the percentage of positive cases is growing compared to previous days or weeks, he said. According to him, that's a warning of a "high-risk situation."
Dr. George Rutherford, an epidemiologist at University of California at San Francisco, offered this tip: If the percentage of positive tests steadily stays under 8 percent, that's generally a good sign.
There's one more caveat about case counts. It takes an average of a week for someone to be infected with COVID-19, develop symptoms, and get tested, Dr. Rutherford said. It can take an additional several days for those test results to be reported to the county health department. This means that case numbers don't represent infections happening right now, but instead are a picture of the state of the pandemic more than a week ago.
Hospitalizations: Focus on Current Statistics
You should be able to find numbers about how many people in your community are currently hospitalized – or have been hospitalized – with diagnoses of COVID-19. But experts say these numbers aren't especially revealing unless you're able to see the number of new hospitalizations over time and track whether they're rising or falling. This number often isn't publicly available, however.
If new hospitalizations are increasing, "you may want to react by being more careful yourself."
And there's an important caveat: "The problem with hospitalizations is that they do lag," UC San Francisco's Dr. Martin said, since it takes time for someone to become ill enough to need to be hospitalized. "They tell you how much virus was being transmitted in your community 2 or 2.5 weeks ago."
Also, he said, people should be cautious about comparing new hospitalization rates between communities unless they're adjusted to account for the number of more-vulnerable older people.
Still, if new hospitalizations are increasing, he said, "you may want to react by being more careful yourself."
Deaths: They're an Even More Delayed Headline
Cable news networks obsessively track the number of coronavirus deaths nationwide, and death counts for every county in the country are available online. Local health departments and media websites may provide charts tracking the growth in deaths over time in your community.
But while death rates offer insight into the disease's horrific toll, they're not useful as an instant snapshot of the pandemic in your community because severely ill patients are typically sick for weeks. Instead, think of them as a delayed headline.
"These numbers don't tell you what's happening today. They tell you how much virus was being transmitted 3-4 weeks ago," Dr. Martin said.
'Reproduction Value': It May Be Revealing
You're not likely to find an available "reproduction value" for your community, but it is available for your state and may be useful.
A reproduction value, also known as R0 or R-naught, "tells us how many people on average we expect will be infected from a single case if we don't take any measures to intervene and if no one has been infected before," said Boston University's Murray.
As The New York Times explained, "R0 is messier than it might look. It is built on hard science, forensic investigation, complex mathematical models — and often a good deal of guesswork. It can vary radically from place to place and day to day, pushed up or down by local conditions and human behavior."
It may be impossible to find the R0 for your community. However, a website created by data specialists is providing updated estimates of a related number -- effective reproduction number, or Rt – for each state. (The R0 refers to how infectious the disease is in general and if precautions aren't taken. The Rt measures its infectiousness at a specific time – the "t" in Rt.) The site is at rt.live.
"The main thing to look at is whether the number is bigger than 1, meaning the outbreak is currently growing in your area, or smaller than 1, meaning the outbreak is currently decreasing in your area," Murray said. "It's also important to remember that this number depends on the prevention measures your community is taking. If the Rt is estimated to be 0.9 in your area and you are currently under lockdown, then to keep it below 1 you may need to remain under lockdown. Relaxing the lockdown could mean that Rt increases above 1 again."
"Whether they're on the upswing or downswing, no state is safe enough to ignore the precautions about mask wearing and social distancing."
Keep in mind that you can still become infected even if an outbreak in your community appears to be slowing. Low risk doesn't mean no risk.
Putting It All Together: Why the Numbers Matter
So you've reviewed COVID-19 statistics in your community. Now what?
Dr. Wilson suggests using the data to remind yourself that the coronavirus pandemic "is still out there. You need to take it seriously and continue precautions," he said. "Whether they're on the upswing or downswing, no state is safe enough to ignore the precautions about mask wearing and social distancing. 'My state is doing well, no one I know is sick, is it time to have a dinner party?' No."
He also recommends that laypeople avoid tracking COVID-19 statistics every day. "Check in once a week or twice a month to see how things are going," he suggested. "Don't stress too much. Just let it remind you to put that mask on before you get out of your car [and are around others]."