Scientists Just Started Testing a New Class of Drugs to Slow--and Even Reverse--Aging

Eliminating "zombie-like" cells, called senescent cells, may hold the key to slowing aging and its chronic diseases.
Imagine reversing the processes of aging. It's an age-old quest, and now a study from the Mayo Clinic may be the first ray of light in the dawn of that new era.
The immune system can handle a certain amount of senescence, but that capacity declines with age.
The small preliminary report, just nine patients, primarily looked at the safety and tolerability of the compounds used. But it also showed that a new class of small molecules called senolytics, which has proven to reverse markers of aging in animal studies, can work in humans.
Aging is a relentless assault of chronic diseases including Alzheimer's, cardiovascular disease, diabetes, and frailty. Developing one chronic condition strongly predicts the rapid onset of another. They pile on top of each other and impede the body's ability to respond to the next challenge.
"Potentially, by targeting fundamental aging processes, it may be possible to delay or prevent or alleviate multiple age-related conditions and many diseases as a group, instead of one at a time," says James Kirkland, the Mayo Clinic physician who led the study and is a top researcher in the growing field of geroscience, the biology of aging.
Getting Rid of "Zombie" Cells
One element common to many of the diseases is senescence, a kind of limbo or zombie-like state where cells no longer divide or perform many regular functions, but they don't die. Senescence is thought to be beneficial in that it inhibits the cancerous proliferation of cells. But in aging, the senescent cells still produce molecules that create inflammation both locally and throughout the body. It is a cycle that feeds upon itself, slowly ratcheting down normal body function and health.
Disease and harmful stimuli like radiation to treat cancer can also generate senescence, which is why young cancer patients seem to experience earlier and more rapid aging. The immune system can handle a certain amount of senescence, but that capacity declines with age. There also appears to be a threshold effect, a tipping point where senescence becomes a dominant factor in aging.
Kirkland's team used an artificial intelligence approach called machine learning to look for cell signaling networks that keep senescent cells from dying. To date, researchers have identified at least eight such signaling networks, some of which seem to be unique to a particular type of cell or tissue, but others are shared or overlap.
Then a computer search identified molecules known to disrupt these signaling pathways "and allow cells that are fully senescent to kill themselves," he explains. The process is a bit like looking for the right weapons in a video game to wipe out lingering zombie cells. But instead of swords, guns, and grenades, the list of biological tools so far includes experimental molecules, approved drugs, and natural supplements.
Treatment
"We found early on that targeting single components of those networks will only kill a very small minority of senescent cells or senescent cell types," says Kirkland. "So instead of going after one drug-one target-one disease, we're going after networks with combinations of drugs or drugs that have multiple targets. And we're going after every age-related disease."
The FDA is grappling with guidance for researchers wanting to conduct clinical trials on something as broad as aging rather than a single disease.
The large number of potential senolytic (i.e. zombie-neutralizing) compounds they identified allowed Kirkland to be choosy, "purposefully selecting drugs where the side effects profile was good...and with short elimination half-lives." The hit and run approach meant they didn't have to worry about maintaining a steady state of drugs in the body for an extended period of time. Some of the compounds they selected need only a half hour exposure to trigger the dying process in senescent cells, which can then take several days.
Work in mice has already shown impressive results in reversing diabetes, weight gain, Alzheimer's, cardiovascular disease and other conditions using senolytic agents.
That led to Kirkland's pilot study in humans with diabetes-related kidney disease using a three-day regimen of dasatinib, a kinase inhibitor first approved in 2006 to treat some forms of blood cancer, and quercetin, a flavonoid found in many plants and sold as a food supplement.
The combination was safe and well tolerated; it reduced the number of senescent cells in the belly fat of patients and restored their normal function, according to results published in September in the journal EBioMedicine. This preliminary paper was based on 9 patients in an ongoing study of 30 patients.
Kirkland cautions that these are initial and incomplete findings looking primarily at safety issues, not effectiveness. There is still much to be learned about the use of senolytics, starting with proof that they actually provide clinical benefit, and against what chronic conditions. The drug combinations, doses, duration, and frequency, not to mention potential risks all must be worked out. Additional studies of other diseases are being developed.
What's Next
Ron Kohanski, a senior administrator at the NIH National Institute on Aging (NIA), says the field of senolytics is so new that there isn't even a consensus on how to identify a senescent cell, and the FDA is grappling with guidance for researchers wanting to conduct clinical trials on something as broad as aging rather than a single disease.
Intellectual property concerns may temper the pharmaceutical industry's interest in developing senolytics to treat chronic diseases of aging. It looks like many mix-and-match combinations are possible, and many of the potential molecules identified so far are found in nature or are drugs whose patents have or will soon expire. So the ability to set high prices for such future drugs, and hence the willingness to spend money on expensive clinical trials, may be limited.
Still, Kohanski believes the field can move forward quickly because it often will include products that are already widely used and have a known safety profile. And approaches like Kirkland's hit and run strategy will minimize potential exposure and risk.
He says the NIA is going to support a number of clinical trials using these new approaches. Pharmaceutical companies may feel that they can develop a unique part of a senolytic combination regimen that will justify their investment. And if they don't, countries with socialized medicine may take the lead in supporting such research with the goal of reducing the costs of treating aging patients.
Nobel Prize goes to technology for mRNA vaccines
Katalin Karikó, pictured, and Drew Weissman won the Nobel Prize for advances in mRNA research that led to the first Covid vaccines.
When Drew Weissman received a call from Katalin Karikó in the early morning hours this past Monday, he assumed his longtime research partner was calling to share a nascent, nagging idea. Weissman, a professor of medicine at the Perelman School of Medicine at the University of Pennsylvania, and Karikó, a professor at Szeged University and an adjunct professor at UPenn, both struggle with sleep disturbances. Thus, middle-of-the-night discourses between the two, often over email, has been a staple of their friendship. But this time, Karikó had something more pressing and exciting to share: They had won the 2023 Nobel Prize in Physiology or Medicine.
The work for which they garnered the illustrious award and its accompanying $1,000,000 cash windfall was completed about two decades ago, wrought through long hours in the lab over many arduous years. But humanity collectively benefited from its life-saving outcome three years ago, when both Moderna and Pfizer/BioNTech’s mRNA vaccines against COVID were found to be safe and highly effective at preventing severe disease. Billions of doses have since been given out to protect humans from the upstart viral scourge.
“I thought of going somewhere else, or doing something else,” said Katalin Karikó. “I also thought maybe I’m not good enough, not smart enough. I tried to imagine: Everything is here, and I just have to do better experiments.”
Unlocking the power of mRNA
Weissman and Karikó unlocked mRNA vaccines for the world back in the early 2000s when they made a key breakthrough. Messenger RNA molecules are essentially instructions for cells’ ribosomes to make specific proteins, so in the 1980s and 1990s, researchers started wondering if sneaking mRNA into the body could trigger cells to manufacture antibodies, enzymes, or growth agents for protecting against infection, treating disease, or repairing tissues. But there was a big problem: injecting this synthetic mRNA triggered a dangerous, inflammatory immune response resulting in the mRNA’s destruction.
While most other researchers chose not to tackle this perplexing problem to instead pursue more lucrative and publishable exploits, Karikó stuck with it. The choice sent her academic career into depressing doldrums. Nobody would fund her work, publications dried up, and after six years as an assistant professor at the University of Pennsylvania, Karikó got demoted. She was going backward.
“I thought of going somewhere else, or doing something else,” Karikó told Stat in 2020. “I also thought maybe I’m not good enough, not smart enough. I tried to imagine: Everything is here, and I just have to do better experiments.”
A tale of tenacity
Collaborating with Drew Weissman, a new professor at the University of Pennsylvania, in the late 1990s helped provide Karikó with the tenacity to continue. Weissman nurtured a goal of developing a vaccine against HIV-1, and saw mRNA as a potential way to do it.
“For the 20 years that we’ve worked together before anybody knew what RNA is, or cared, it was the two of us literally side by side at a bench working together,” Weissman said in an interview with Adam Smith of the Nobel Foundation.
In 2005, the duo made their 2023 Nobel Prize-winning breakthrough, detailing it in a relatively small journal, Immunity. (Their paper was rejected by larger journals, including Science and Nature.) They figured out that chemically modifying the nucleoside bases that make up mRNA allowed the molecule to slip past the body’s immune defenses. Karikó and Weissman followed up that finding by creating mRNA that’s more efficiently translated within cells, greatly boosting protein production. In 2020, scientists at Moderna and BioNTech (where Karikó worked from 2013 to 2022) rushed to craft vaccines against COVID, putting their methods to life-saving use.
The future of vaccines
Buoyed by the resounding success of mRNA vaccines, scientists are now hurriedly researching ways to use mRNA medicine against other infectious diseases, cancer, and genetic disorders. The now ubiquitous efforts stand in stark contrast to Karikó and Weissman’s previously unheralded struggles years ago as they doggedly worked to realize a shared dream that so many others shied away from. Katalin Karikó and Drew Weissman were brave enough to walk a scientific path that very well could have ended in a dead end, and for that, they absolutely deserve their 2023 Nobel Prize.
This article originally appeared on Big Think, home of the brightest minds and biggest ideas of all time.

Scientists turn pee into power in Uganda
With conventional fuel cells as their model, researchers learned to use similar chemical reactions to make a fuel from microbes in pee.
At the edge of a dirt road flanked by trees and green mountains outside the town of Kisoro, Uganda, sits the concrete building that houses Sesame Girls School, where girls aged 11 to 19 can live, learn and, at least for a while, safely use a toilet. In many developing regions, toileting at night is especially dangerous for children. Without electrical power for lighting, kids may fall into the deep pits of the latrines through broken or unsteady floorboards. Girls are sometimes assaulted by men who hide in the dark.
For the Sesame School girls, though, bright LED lights, connected to tiny gadgets, chased the fears away. They got to use new, clean toilets lit by the power of their own pee. Some girls even used the light provided by the latrines to study.
Urine, whether animal or human, is more than waste. It’s a cheap and abundant resource. Each day across the globe, 8.1 billion humans make 4 billion gallons of pee. Cows, pigs, deer, elephants and other animals add more. By spending money to get rid of it, we waste a renewable resource that can serve more than one purpose. Microorganisms that feed on nutrients in urine can be used in a microbial fuel cell that generates electricity – or "pee power," as the Sesame girls called it.
Plus, urine contains water, phosphorus, potassium and nitrogen, the key ingredients plants need to grow and survive. Human urine could replace about 25 percent of current nitrogen and phosphorous fertilizers worldwide and could save water for gardens and crops. The average U.S. resident flushes a toilet bowl containing only pee and paper about six to seven times a day, which adds up to about 3,500 gallons of water down per year. Plus cows in the U.S. produce 231 gallons of the stuff each year.
Pee power
A conventional fuel cell uses chemical reactions to produce energy, as electrons move from one electrode to another to power a lightbulb or phone. Ioannis Ieropoulos, a professor and chair of Environmental Engineering at the University of Southampton in England, realized the same type of reaction could be used to make a fuel from microbes in pee.
Bacterial species like Shewanella oneidensis and Pseudomonas aeruginosa can consume carbon and other nutrients in urine and pop out electrons as a result of their digestion. In a microbial fuel cell, one electrode is covered in microbes, immersed in urine and kept away from oxygen. Another electrode is in contact with oxygen. When the microbes feed on nutrients, they produce the electrons that flow through the circuit from one electrod to another to combine with oxygen on the other side. As long as the microbes have fresh pee to chomp on, electrons keep flowing. And after the microbes are done with the pee, it can be used as fertilizer.
These microbes are easily found in wastewater treatment plants, ponds, lakes, rivers or soil. Keeping them alive is the easy part, says Ieropoulos. Once the cells start producing stable power, his group sequences the microbes and keeps using them.
Like many promising technologies, scaling these devices for mass consumption won’t be easy, says Kevin Orner, a civil engineering professor at West Virginia University. But it’s moving in the right direction. Ieropoulos’s device has shrunk from the size of about three packs of cards to a large glue stick. It looks and works much like a AAA battery and produce about the same power. By itself, the device can barely power a light bulb, but when stacked together, they can do much more—just like photovoltaic cells in solar panels. His lab has produced 1760 fuel cells stacked together, and with manufacturing support, there’s no theoretical ceiling, he says.
Although pure urine produces the most power, Ieropoulos’s devices also work with the mixed liquids of the wastewater treatment plants, so they can be retrofit into urban wastewater utilities.
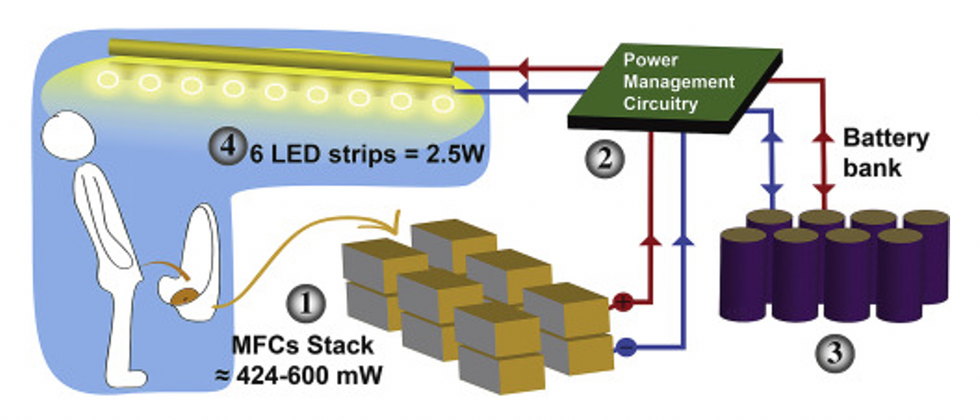
This image shows how the pee-powered system works. Pee feeds bacteria in the stack of fuel cells (1), which give off electrons (2) stored in parallel cylindrical cells (3). These cells are connected to a voltage regulator (4), which smooths out the electrical signal to ensure consistent power to the LED strips lighting the toilet.
Courtesy Ioannis Ieropoulos
Key to the long-term success of any urine reclamation effort, says Orner, is avoiding what he calls “parachute engineering”—when well-meaning scientists solve a problem with novel tech and then abandon it. “The way around that is to have either the need come from the community or to have an organization in a community that is committed to seeing a project operate and maintained,” he says.
Success with urine reclamation also depends on the economy. “If energy prices are low, it may not make sense to recover energy,” says Orner. “But right now, fertilizer prices worldwide are generally pretty high, so it may make sense to recover fertilizer and nutrients.” There are obstacles, too, such as few incentives for builders to incorporate urine recycling into new construction. And any hiccups like leaks or waste seepage will cost builders money and reputation. Right now, Orner says, the risks are just too high.
Despite the challenges, Ieropoulos envisions a future in which urine is passed through microbial fuel cells at wastewater treatment plants, retrofitted septic tanks, and building basements, and is then delivered to businesses to use as agricultural fertilizers. Although pure urine produces the most power, Ieropoulos’s devices also work with the mixed liquids of the wastewater treatment plants, so they can be retrofitted into urban wastewater utilities where they can make electricity from the effluent. And unlike solar cells, which are a common target of theft in some areas, nobody wants to steal a bunch of pee.
When Ieropoulos’s team returned to wrap up their pilot project 18 months later, the school’s director begged them to leave the fuel cells in place—because they made a major difference in students’ lives. “We replaced it with a substantial photovoltaic panel,” says Ieropoulos, They couldn’t leave the units forever, he explained, because of intellectual property reasons—their funders worried about theft of both the technology and the idea. But the photovoltaic replacement could be stolen, too, leaving the girls in the dark.
The story repeated itself at another school, in Nairobi, Kenya, as well as in an informal settlement in Durban, South Africa. Each time, Ieropoulos vowed to return. Though the pandemic has delayed his promise, he is resolute about continuing his work—it is a moral and legal obligation. “We've made a commitment to ourselves and to the pupils,” he says. “That's why we need to go back.”
Urine as fertilizer
Modern day industrial systems perpetuate the broken cycle of nutrients. When plants grow, they use up nutrients the soil. We eat the plans and excrete some of the nutrients we pass them into rivers and oceans. As a result, farmers must keep fertilizing the fields while our waste keeps fertilizing the waterways, where the algae, overfertilized with nitrogen, phosphorous and other nutrients grows out of control, sucking up oxygen that other marine species need to live. Few global communities remain untouched by the related challenges this broken chain create: insufficient clean water, food, and energy, and too much human and animal waste.
The Rich Earth Institute in Vermont runs a community-wide urine nutrient recovery program, which collects urine from homes and businesses, transports it for processing, and then supplies it as fertilizer to local farms.
One solution to this broken cycle is reclaiming urine and returning it back to the land. The Rich Earth Institute in Vermont is one of several organizations around the world working to divert and save urine for agricultural use. “The urine produced by an adult in one day contains enough fertilizer to grow all the wheat in one loaf of bread,” states their website.
Notably, while urine is not entirely sterile, it tends to harbor fewer pathogens than feces. That’s largely because urine has less organic matter and therefore less food for pathogens to feed on, but also because the urinary tract and the bladder have built-in antimicrobial defenses that kill many germs. In fact, the Rich Earth Institute says it’s safe to put your own urine onto crops grown for home consumption. Nonetheless, you’ll want to dilute it first because pee usually has too much nitrogen and can cause “fertilizer burn” if applied straight without dilution. Other projects to turn urine into fertilizer are in progress in Niger, South Africa, Kenya, Ethiopia, Sweden, Switzerland, The Netherlands, Australia, and France.
Eleven years ago, the Institute started a program that collects urine from homes and businesses, transports it for processing, and then supplies it as fertilizer to local farms. By 2021, the program included 180 donors producing over 12,000 gallons of urine each year. This urine is helping to fertilize hay fields at four partnering farms. Orner, the West Virginia professor, sees it as a success story. “They've shown how you can do this right--implementing it at a community level scale."

