Man Who Got the First Fecal Transplant to Cure Melanoma Shares His Experience

Jamie Rettinger with his now fiance Amie Purnel-Davis, who helped him through the clinical trial.
Jamie Rettinger was still in his thirties when he first noticed a tiny streak of brown running through the thumbnail of his right hand. It slowly grew wider and the skin underneath began to deteriorate before he went to a local dermatologist in 2013. The doctor thought it was a wart and tried scooping it out, treating the affected area for three years before finally removing the nail bed and sending it off to a pathology lab for analysis.
"I have some bad news for you; what we removed was a five-millimeter melanoma, a cancerous tumor that often spreads," Jamie recalls being told on his return visit. "I'd never heard of cancer coming through a thumbnail," he says. None of his doctors had ever mentioned it either. "I just thought I was being treated for a wart." But nothing was healing and it continued to bleed.
A few months later a surgeon amputated the top half of his thumb. Lymph node biopsy tested negative for spread of the cancer and when the bandages finally came off, Jamie thought his medical issues were resolved.
Melanoma is the deadliest form of skin cancer. About 85,000 people are diagnosed with it each year in the U.S. and more than 8,000 die of the cancer when it spreads to other parts of the body, according to the Centers for Disease Control and Prevention (CDC).
There are two peaks in diagnosis of melanoma; one is in younger women ages 30-40 and often is tied to past use of tanning beds; the second is older men 60+ and is related to outdoor activity from farming to sports. Light-skinned people have a twenty-times greater risk of melanoma than do people with dark skin.
"When I graduated from medical school, in 2005, melanoma was a death sentence" --Diwakar Davar.
Jamie had a follow up PET scan about six months after his surgery. A suspicious spot on his lung led to a biopsy that came back positive for melanoma. The cancer had spread. Treatment with a monoclonal antibody (nivolumab/Opdivo®) didn't prove effective and he was referred to the UPMC Hillman Cancer Center in Pittsburgh, a four-hour drive from his home in western Ohio.
An alternative monoclonal antibody treatment brought on such bad side effects, diarrhea as often as 15 times a day, that it took more than a week of hospitalization to stabilize his condition. The only options left were experimental approaches in clinical trials.
Early research
"When I graduated from medical school, in 2005, melanoma was a death sentence" with a cure rate in the single digits, says Diwakar Davar, 39, an oncologist at UPMC Hillman Cancer Center who specializes in skin cancer. That began to change in 2010 with introduction of the first immunotherapies, monoclonal antibodies, to treat cancer. The antibodies attach to PD-1, a receptor on the surface of T cells of the immune system and on cancer cells. Antibody treatment boosted the melanoma cure rate to about 30 percent. The search was on to understand why some people responded to these drugs and others did not.
At the same time, there was a growing understanding of the role that bacteria in the gut, the gut microbiome, plays in helping to train and maintain the function of the body's various immune cells. Perhaps the bacteria also plays a role in shaping the immune response to cancer therapy.
One clue came from genetically identical mice. Animals ordered from different suppliers sometimes responded differently to the experiments being performed. That difference was traced to different compositions of their gut microbiome; transferring the microbiome from one animal to another in a process known as fecal transplant (FMT) could change their responses to disease or treatment.
When researchers looked at humans, they found that the patients who responded well to immunotherapies had a gut microbiome that looked like healthy normal folks, but patients who didn't respond had missing or reduced strains of bacteria.
Davar and his team knew that FMT had a very successful cure rate in treating the gut dysbiosis of Clostridioides difficile, a persistant intestinal infection, and they wondered if a fecal transplant from a patient who had responded well to cancer immunotherapy treatment might improve the cure rate of patients who did not originally respond to immunotherapies for melanoma.
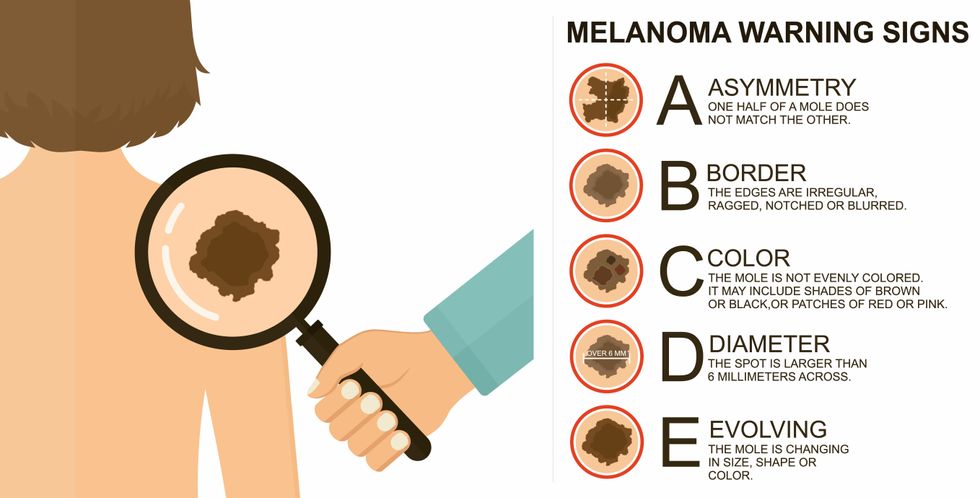
The ABCDE of melanoma detection
Adobe Stock
Clinical trial
"It was pretty weird, I was totally blasted away. Who had thought of this?" Jamie first thought when the hypothesis was explained to him. But Davar's explanation that the procedure might restore some of the beneficial bacterial his gut was lacking, convinced him to try. He quickly signed on in October 2018 to be the first person in the clinical trial.
Fecal donations go through the same safety procedures of screening for and inactivating diseases that are used in processing blood donations to make them safe for transfusion. The procedure itself uses a standard hollow colonoscope designed to screen for colon cancer and remove polyps. The transplant is inserted through the center of the flexible tube.
Most patients are sedated for procedures that use a colonoscope but Jamie doesn't respond to those drugs: "You can't knock me out. I was watching them on the TV going up my own butt. It was kind of unreal at that point," he says. "There were about twelve people in there watching because no one had seen this done before."
A test two weeks after the procedure showed that the FMT had engrafted and the once-missing bacteria were thriving in his gut. More importantly, his body was responding to another monoclonal antibody (pembrolizumab/Keytruda®) and signs of melanoma began to shrink. Every three months he made the four-hour drive from home to Pittsburgh for six rounds of treatment with the antibody drug.
"We were very, very lucky that the first patient had a great response," says Davar. "It allowed us to believe that even though we failed with the next six, we were on the right track. We just needed to tweak the [fecal] cocktail a little better" and enroll patients in the study who had less aggressive tumor growth and were likely to live long enough to complete the extensive rounds of therapy. Six of 15 patients responded positively in the pilot clinical trial that was published in the journal Science.
Davar believes they are beginning to understand the biological mechanisms of why some patients initially do not respond to immunotherapy but later can with a FMT. It is tied to the background level of inflammation produced by the interaction between the microbiome and the immune system. That paper is not yet published.
Surviving cancer
It has been almost a year since the last in his series of cancer treatments and Jamie has no measurable disease. He is cautiously optimistic that his cancer is not simply in remission but is gone for good. "I'm still scared every time I get my scans, because you don't know whether it is going to come back or not. And to realize that it is something that is totally out of my control."
"It was hard for me to regain trust" after being misdiagnosed and mistreated by several doctors he says. But his experience at Hillman helped to restore that trust "because they were interested in me, not just fixing the problem."
He is grateful for the support provided by family and friends over the last eight years. After a pause and a sigh, the ruggedly built 47-year-old says, "If everyone else was dead in my family, I probably wouldn't have been able to do it."
"I never hesitated to ask a question and I never hesitated to get a second opinion." But Jamie acknowledges the experience has made him more aware of the need for regular preventive medical care and a primary care physician. That person might have caught his melanoma at an earlier stage when it was easier to treat.
Davar continues to work on clinical studies to optimize this treatment approach. Perhaps down the road, screening the microbiome will be standard for melanoma and other cancers prior to using immunotherapies, and the FMT will be as simple as swallowing a handful of freeze-dried capsules off the shelf rather than through a colonoscopy. Earlier this year, the Food and Drug Administration approved the first oral fecal microbiota product for C. difficile, hopefully paving the way for more.
An older version of this hit article was first published on May 18, 2021
Living with someone changes your microbiome, new research shows
For the first time, research has shown that bacteria of the microbiome are transmitted between many individuals, not just infants and their mothers, in ways that can’t be explained by having the same diet or geography.
Some roommate frustration can be expected, whether it’s a sink piled high with crusty dishes or crumbs where a clean tabletop should be. Now, research suggests a less familiar issue: person-to-person transmission of shared bacterial strains in our gut and oral microbiomes. For the first time, the lab of Nicola Segata, a professor of genetics and computational biology at the University of Trento, located in Italy, has shown that bacteria of the microbiome are transmitted between many individuals, not just infants and their mothers, in ways that can’t be explained by their shared diet or geography.
It’s a finding with wide-ranging implications, yet frustratingly few predictable outcomes. Our microbiomes are an ever-growing and changing collection of helpful and harmful bacteria that we begin to accumulate the moment we’re born, but experts are still struggling to unravel why and how bacteria from one person’s gut or mouth become established in another person’s microbiome, as opposed to simply passing through.
“If we are looking at the overall species composition of the microbiome, then there is an effect of age of course, and many other factors,” Segata says. “But if we are looking at where our strains are coming from, 99 percent of them are only present in other people’s guts. They need to come from other guts.”
If we could better understand this process, we might be able to control and use it; perhaps hospital patients could avoid infections from other patients when their microbiome is depleted by antibiotics and their immune system is weakened, for example. But scientists are just beginning to link human microbiomes with various ailments. Growing evidence shows that our microbiomes steer our long-term health, impacting conditions like obesity, irritable bowel syndrome, type 2 diabetes, and cancer.
Previous work from Segata’s lab and others illuminated the ways bacteria are passed from mothers to infants during the first few months of life during vaginal birth, breastfeeding and other close contact. And scientists have long known that people in close proximity tend to share bacteria. But the factors related to that overlap, such as genetics and diet, were unclear, especially outside the mother-baby dyad.
“If we look at strain sharing between a mother and an infant at five years of age, for example, we cannot really tell which was due to transmission at birth and which is due to continued transmission because of contact,” Segata says. Experts hypothesized that they could be caused by bacterial similarities in the environment itself, genetics, or bacteria from shared foods that colonized the guts of people in close contact.
Strain sharing was highest in mother-child pairs, with 96 percent of them sharing strains, and only slightly lower in members of shared households, at 95 percent.
In Italy, researchers led by Mireia Valles-Colomer, including Segata, hoped to unravel this mystery. They compared data from 9,715 stool and saliva samples in 31 genomic datasets with existing metadata. Scientists zoomed in on variations in each bacterial strain down to the individual level. They examined not only mother-child pairs, but people living in the same household, adult twins, and people living in the same village in a level of detail that wasn’t possible before, due to its high cost and difficulties in retrieving data about interactions between individuals, Segata explained.
“This paper is, with high granularity, quantifying the percent sharing that you expect between different types of social interactions, controlling for things like genetics and diet,” Gibbons says. Strain sharing was highest in mother-child pairs, with 96 percent of them sharing strains, and only slightly lower in members of shared households, at 95 percent. And at least half of the mother-infant pairs shared 30 percent of their strains; the median was 12 percent among people in shared households. Yet, there was no sharing among eight percent of adult twins who lived separately, and 16 percent of people within villages who resided in different households. The results were published in Nature.
It’s not a regional phenomenon. Although the types of bacterial strains varied depending on whether people lived in western and eastern nations — datasets were drawn from 20 countries on five continents — the patterns of sharing were much the same. To establish these links, scientists focused on individual variations in shared bacterial strains, differences that create unique bacterial “fingerprints” in each person, while controlling for variables like diet, demonstrating that the bacteria had been transmitted between people and were not the result of environmental similarities.
The impact of this bacterial sharing isn’t clear, but shouldn’t be viewed with trepidation, according to Sean Gibbons, a microbiome scientist at the nonprofit Institute for Systems Biology.
“The vast majority of these bugs are actually either benign or beneficial to our health, and the fact that we're swapping and sharing them and that we can take someone else's strain and supplement or better diversify our own little garden is not necessarily a bad thing,” he says.
"There are hundreds of billions of dollars of investment capital moving into these microbiome therapeutic companies; bugs as drugs, so to speak,” says Sean Gibbons, a microbiome scientist at the Institute for Systems Biology.
Everyday habits like exercising and eating vegetables promote a healthy, balanced gut microbiome, which is linked to better metabolic and immune function, and fewer illnesses. While many people’s microbiomes contain bacteria like C. diff or E. coli, these bacteria don’t cause diseases in most cases because they’re present in low levels. But a microbiome that’s been wiped out by, say, antibiotics, may no longer keep these bacteria in check, allowing them to proliferate and make us sick.
“A big challenge in the microbiome field is being able to rationally predict whether, if you're exposed to a particular bug, it will stick in the context of your specific microbiome,” Gibbons says.
Gibbons predicts that explorations of microbe-based therapeutics will be “exploding” in the coming decades. “There are hundreds of billions of dollars of investment capital moving into these microbiome therapeutic companies; bugs as drugs, so to speak,” he says. Rather than taking a mass-marketed probiotic, a precise understanding of an individual’s microbiome could help target the introduction of just the right bacteria at just the right time to prevent or treat a particular illness.
Because the current study did not differentiate between different types of contact or relationships among household members sharing bacterial strains or determine the direction of transmission, Segata says his current project is examining children in daycare settings and tracking their microbiomes over time to understand the role genetics and everyday interactions play in the level of transmission that occurs.
This relatively newfound ability to trace bacterial variants to minute levels has unlocked the chance for scientists to untangle when and how bacteria leap from one microbiome to another. As researchers come to better understand the factors that permit a strain to establish itself within a microbiome, they could uncover new strategies to control these microbes, harnessing the makeup of each microbiome to help people to resist life-altering medical conditions.
Are the gains from gain-of-function research worth the risks?
Gain-of-function research can make pathogens more infectious and deadly. It also enables scientists to prepare remedies in advance.
Scientists have long argued that gain-of-function research, which can make viruses and other infectious agents more contagious or more deadly, was necessary to develop therapies and vaccines to counter the pathogens in case they were used for biological warfare. As the SARS-CoV-2 origins are being investigated, one prominent theory suggests it had leaked from a biolab that conducted gain-of-function research, causing a global pandemic that claimed nearly 6.9 million lives. Now some question the wisdom of engaging in this type of research, stating that the risks may far outweigh the benefits.
“Gain-of-function research means genetically changing a genome in a way that might enhance the biological function of its genes, such as its transmissibility or the range of hosts it can infect,” says George Church, professor of genetics at Harvard Medical School. This can occur through direct genetic manipulation as well as by encouraging mutations while growing successive generations of micro-organism in culture. “Some of these changes may impact pathogenesis in a way that is hard to anticipate in advance,” Church says.
In the wake of the global pandemic, the pros and cons of gain-of-function research are being fiercely debated. Some scientists say this type of research is vital for preventing future pandemics or for preparing for bioweapon attacks. Others consider it another disaster waiting to happen. The Government Accounting Office issued a report charging that a framework developed by the U.S. Department of Health & Human Services (HHS) provided inadequate oversight of this potentially deadly research. There’s a movement to stop it altogether. In January, the Viral Gain-of-Function Research Moratorium Act (S. 81) was introduced into the Senate to cease awarding federal research funding to institutions doing gain-of-function studies.
While testifying before the House COVID Origins Select Committee on March 8th, Robert Redfield, former director of the U.S. Centers for Disease Control and Prevention, said that COVID-19 may have resulted from an accidental lab leak involving gain-of-function research. Redfield said his conclusion is based upon the “rapid and high infectivity for human-to-human transmission, which then predicts the rapid evolution of new variants.”
“It is a very, very, very small subset of life science research that could potentially generate a potential pandemic pathogen,” said Gerald Parker, associate dean for Global One Health at Texas A&M University.
“In my opinion,” Redfield continues, “the COVID-19 pandemic presents a case study on the potential dangers of such research. While many believe that gain-of-function research is critical to get ahead of viruses by developing vaccines, in this case, I believe that was the exact opposite.” Consequently, Redfield called for a moratorium on gain-of-function research until there is consensus about the value of such risky science.
What constitutes risky?
The Federal Select Agent Program lists 68 specific infectious agents as risky because they are either very contagious or very deadly. In order to work with these 68 agents, scientists must register with the federal government. Meanwhile, research on deadly pathogens that aren’t easily transmitted, or pathogens that are quite contagious but not deadly, can be conducted without such oversight. “If you’re not working with select agents, you’re not required to register the research with the federal government,” says Gerald Parker, associate dean for Global One Health at Texas A&M University. But the 68-item list may not have everything that could possibly become dangerous or be engineered to be dangerous, thus escaping the government’s scrutiny—an issue that new regulations aim to address.
In January 2017, the White House Office of Science and Technology Policy (OSTP) issued additional guidance. It required federal departments and agencies to follow a series of steps when reviewing proposed research that could create, transfer, or use potential pandemic pathogens resulting from the enhancement of a pathogen’s transmissibility or virulence in humans.
In defining risky pathogens, OSTP included viruses that were likely to be highly transmissible and highly virulent, and thus very deadly. The Proposed Biosecurity Oversight Framework for the Future of Science, outlined in 2023, broadened the scope to require federal review of research “that is reasonably anticipated to enhance the transmissibility and/or virulence of any pathogen” likely to pose a threat to public health, health systems or national security. Those types of experiments also include the pathogens’ ability to evade vaccines or therapeutics, or diagnostic detection.
However, Parker says that dangers of generating a pandemic-level germ are tiny. “It is a very, very, very small subset of life science research that could potentially generate a potential pandemic pathogen.” Since gain-of-function guidelines were first issued in 2017, only three such research projects have met those requirements for HHS review. They aimed to study influenza and bird flu. Only two of those projects were funded, according to the NIH Office of Science Policy. For context, NIH funded approximately 11,000 of the 54,000 grant applications it received in 2022.
Guidelines governing gain-of-function research are being strengthened, but Church points out they aren’t ideal yet. “They need to be much clearer about penalties and avoiding positive uses before they would be enforceable.”
What do we gain from gain-of-function research?
The most commonly cited reason to conduct gain-of-function research is for biodefense—the government’s ability to deal with organisms that may pose threats to public health.
In the era of mRNA vaccines, the advance preparedness argument may be even less relevant.
“The need to work with potentially dangerous viruses is central to our preparedness,” Parker says. “It’s essential that we know and understand the basic biology, microbiology, etc. of some of these dangerous pathogens.” That includes increasing our knowledge of the molecular mechanisms by which a virus could become a sustained threat to humans. “Knowing that could help us detect [risks] earlier,” Parker says—and could make it possible to have medical countermeasures, like vaccines and therapeutics, ready.
Most vaccines, however, aren’t affected by this type of research. Essentially, scientists hope they will never need to use it. Moreover, Paul Mango, HSS former deputy chief of staff for policy, and author of the 2022 book Warp Speed, says he believes that in the era of mRNA vaccines, the advance preparedness argument may be even less relevant. “That’s because these vaccines can be developed and produced in less than 12 months, unlike traditional vaccines that require years of development,” he says.
Can better oversight guarantee safety?
Another situation, which Parker calls unnecessarily dangerous, is when regulatory bodies cannot verify that the appropriate biosafety and biosecurity controls are in place.
Gain-of-function studies, Parker points out, are conducted at the basic research level, and they’re performed in high-containment labs. “As long as all the processes, procedures and protocols are followed and there’s appropriate oversight at the institutional and scientific level, it can be conducted safely.”
Globally, there are 69 Biosafety Level 4 (BSL4) labs operating, under construction or being planned, according to recent research from King’s College London and George Mason University for Global BioLabs. Eleven of these 18 high-containment facilities that are planned or under construction are in Asia. Overall, three-quarters of the BSL4 labs are in cities, increasing public health risks if leaks occur.
Researchers say they are confident in the oversight system for BSL4 labs within the U.S. They are less confident in international labs. Global BioLabs’ report concurs. It gives the highest scores for biosafety to industrialized nations, led by France, Australia, Canada, the U.S. and Japan, and the lowest scores to Saudi Arabia, India and some developing African nations. Scores for biosecurity followed similar patterns.
“There are no harmonized international biosafety and biosecurity standards,” Parker notes. That issue has been discussed for at least a decade. Now, in the wake of SARS and the COVID-19 pandemic, scientists and regulators are likely to push for unified oversight standards. “It’s time we got serious about international harmonization of biosafety and biosecurity standards and guidelines,” Parker says. New guidelines are being worked on. The National Science Advisory Board for Biosecurity (NSABB) outlined its proposed recommendations in the document titled Proposed Biosecurity Oversight Framework for the Future of Science.
The debates about whether gain-of-function research is useful or poses unnecessary risks to humanity are likely to rage on for a while. The public too has a voice in this debate and should weigh in by communicating with their representatives in government, or by partaking in educational forums or initiatives offered by universities and other institutions. In the meantime, scientists should focus on improving the research regulations, Parker notes. “We need to continue to look for lessons learned and for gaps in our oversight system,” he says. “That’s what we need to do right now.”