Slowing Aging Could Transform Society As We Know It

A young woman portrayed next to her old counterpart.
People's lives have been getting longer for more than a century. In 1900, in even the wealthiest countries, life expectancy was under 50, according to the World Health Organization. By 2015, the worldwide average was 74, and a girl born in Japan that year could expect to live to 87. Most of that extra lifespan came from improvements in nutrition and sanitation, and the development of vaccines and antibiotics.
People's lives have been getting longer for more than a century. In 1900, in even the wealthiest countries, life expectancy was under 50, according to the World Health Organization. By 2015, the worldwide average was 74, and a girl born in Japan that year could expect to live to 87. Most of that extra lifespan came from improvements in nutrition and sanitation, and the development of vaccines and antibiotics.
The question is, how will slowing aging change society?
But now scientists are trying to move beyond just eliminating the diseases that kill us to actually slowing the aging process itself. By developing new drugs to tackle the underlying mechanisms that make our bodies grow old and frail, researchers hope to give people many more years of healthy life. The question is, how will that change society?
There are several biological mechanisms that affect aging. One involves how cells react when they're damaged. Some die, but others enter a state called senescence, in which they halt their normal growth and send out signals that something's gone wrong. That signaling causes inflammation at the sight of a wound, for instance, and triggers the body's repair processes. Once everything is back to normal, the senescent cells die off and the inflammation fades. But as we age, the machinery for clearing senescent cells becomes less efficient and they begin to pile up. Some researchers think that this accumulation of senescent cells is what causes chronic inflammation, which has been implicated in conditions such as heart disease and diabetes.
The first clinical trial in humans of senolytic drugs is happening now.
In 2015, researchers at the Mayo Clinic in Minnesota and the Scripps Research Institute in Florida tested the first so-called senolytic drugs, which cause senescent cells to die. After the scientists treated mice with a combination of an anti-cancer drug and a plant pigment that can act as an antioxidant, some of the senescent cells shrank away and caused the mouse's heart function to revert to that of a much younger mouse.
"That suggests that senescence isn't just a consequence of aging, it's actually a driver of aging," says Paul Robbins, a professor of molecular medicine at Scripps and one of the researchers involved. Other animal studies have found that reducing the number of senescent cells improves a variety of age-related conditions, such as frailty, diabetes, liver disease, pulmonary fibrosis, and osteoporosis.
Now the same researchers are moving those tests to humans in the first clinical trials of senolytic drugs. In July 2016, the Mayo Clinic launched what may be the first clinical trial of senolytic therapy, studying the effect of the two drugs, called dasatinib and quercetin, on people with chronic kidney disease, which they hope to complete in 2021. Meanwhile Mayo and Scripps researchers have identified six different biochemical pathways that give rise to senescence, along with several drug candidates that target those pathways. Robbins says it's likely that different drugs will work better for different cells in the body.
Would radical life extension lead to moral deterioration, risk aversion, and an abandonment of creativity?
In Robbins' work, treating mice with senolytic drugs has extended their median lifespan—the age at which half the animals in his experiment have died—by about 30 percent, but hasn't extended the maximum lifespan. In other words, the oldest mice treated with the drugs died at the same age as mice who hadn't been treated, but more of the mice who received senolytics lived to that ripe old age. The same may turn out to be true for humans, with more people living to the limits of the lifespan—estimated by some to be about 115—but no one living much longer. On the other hand, Robbins says, it's early days for these therapies, and it may turn out that delaying aging actually does push the limit of life farther out.
Others expect more radical extensions of human life; British gerontologist Aubrey DeGray talks about people living for 1000 years, and people who call themselves transhumanists imagine replacing body parts as they wear out, or merging our minds with computers to make us essentially immortal. Brian Green, an ethicist at Santa Clara University in California, finds that concept horrifying. He fears it would make people value their own lives too highly, demoting other moral goods such as self-sacrifice or concern for the environment. "It kind of lends itself to a moral myopia," he says. "Humans work better if they have a goal beyond their own survival." And people who live for centuries might become averse to risk, because with longer lives they have more to lose if they were to accidentally die, and might be resistant to change, draining the world of creativity.
Most researchers are focused on "extending the 'healthspan,' so that the people who live into their 90s are vigorous and disease-free."
He's not too worried, though, that that's where studies such as the Mayo Clinic's are headed, and supports that sort of research. "Hopefully these things will work, and they'll help us live a little bit longer," Green says, "but the idea of radical life extension where we're going to live indefinitely longer, I think that is very unrealistic."
Most of the researchers working on combatting aging don't, in fact, talk of unlimited lifespans. Rather, they talk about extending the "healthspan," so that the people who live into their 90s are vigorous and disease-free up until nearly the end of their lives.
If scientists can lengthen life while reducing the number of years people suffer with dementia or infirmity, that could be beneficial, says Stephen Post, a professor of medicine and director of the Center for Medical Humanities, Compassionate Care, and Bioethics at Stony Brook University in New York. But even increasing the population of vigorous 90-somethings might have negative implications for society. "What would we do with all these people who are living so long?" he asks. "Would we stop having children? Would we never retire?"
Adding 2.2 healthy years to the U.S. life by delaying aging could benefit the economy by $7.1 trillion over 50 years.
If people keep working well past their 60s, that could mean there would be fewer jobs available for younger people, says Maxwell Mehlman, professor of bioethics at Case Western Reserve University's School of Law in Ohio. Mehlman says society may have to rethink age discrimination laws, which bar firing or refusing to hire people over a certain age, to make room for younger workers. On the other hand, those who choose to retire and live another two or three decades could strain pension and entitlement systems.
But a longer healthspan could reduce costs in the healthcare system, which now are driven disproportionately by older people. Jay Olshansky, an epidemiologist at the University of Illinois at Chicago School of Public Health, has estimated that adding 2.2 healthy years to the U.S. life by delaying aging would benefit the economy by $7.1 trillion over 50 years, as spending on illnesses such as cancer and heart disease drop.
For his part, Robbins says that the scientific conferences in the anti-aging field, which tend to focus on the technical research, should hold more sessions on social and economic impacts. If anti-aging therapies start extending healthy lifespans, as he and other researchers hope they will within a decade or so, society will need to adjust.
Ultimately, it's an extension of health, not just of longevity, that will benefit us. Extra decades of senescence do nobody any good. As Green says, "Nobody wants to live in a nursing home for 1000 years."
Thanks to safety cautions from the COVID-19 pandemic, a strain of influenza has been completely eliminated.
If you were one of the millions who masked up, washed your hands thoroughly and socially distanced, pat yourself on the back—you may have helped change the course of human history.
Scientists say that thanks to these safety precautions, which were introduced in early 2020 as a way to stop transmission of the novel COVID-19 virus, a strain of influenza has been completely eliminated. This marks the first time in human history that a virus has been wiped out through non-pharmaceutical interventions, such as vaccines.
The flu shot, explained
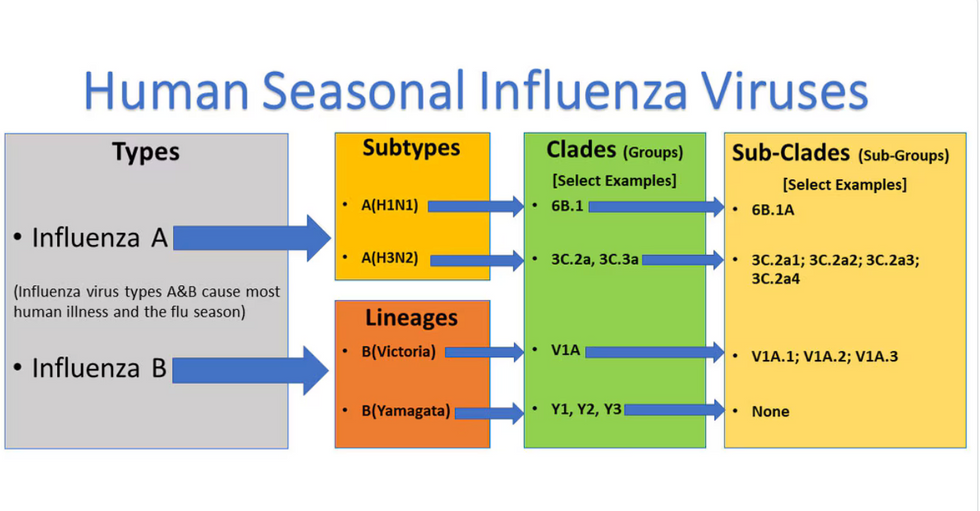
Influenza viruses type A and B are responsible for the majority of human illnesses and the flu season.
Centers for Disease Control
For more than a decade, flu shots have protected against two types of the influenza virus–type A and type B. While there are four different strains of influenza in existence (A, B, C, and D), only strains A, B, and C are capable of infecting humans, and only A and B cause pandemics. In other words, if you catch the flu during flu season, you’re most likely sick with flu type A or B.
Flu vaccines contain inactivated—or dead—influenza virus. These inactivated viruses can’t cause sickness in humans, but when administered as part of a vaccine, they teach a person’s immune system to recognize and kill those viruses when they’re encountered in the wild.
Each spring, a panel of experts gives a recommendation to the US Food and Drug Administration on which strains of each flu type to include in that year’s flu vaccine, depending on what surveillance data says is circulating and what they believe is likely to cause the most illness during the upcoming flu season. For the past decade, Americans have had access to vaccines that provide protection against two strains of influenza A and two lineages of influenza B, known as the Victoria lineage and the Yamagata lineage. But this year, the seasonal flu shot won’t include the Yamagata strain, because the Yamagata strain is no longer circulating among humans.
How Yamagata Disappeared

Flu surveillance data from the Global Initiative on Sharing All Influenza Data (GISAID) shows that the Yamagata lineage of flu type B has not been sequenced since April 2020.
Nature
Experts believe that the Yamagata lineage had already been in decline before the pandemic hit, likely because the strain was naturally less capable of infecting large numbers of people compared to the other strains. When the COVID-19 pandemic hit, the resulting safety precautions such as social distancing, isolating, hand-washing, and masking were enough to drive the virus into extinction completely.
Because the strain hasn’t been circulating since 2020, the FDA elected to remove the Yamagata strain from the seasonal flu vaccine. This will mark the first time since 2012 that the annual flu shot will be trivalent (three-component) rather than quadrivalent (four-component).
Should I still get the flu shot?
The flu shot will protect against fewer strains this year—but that doesn’t mean we should skip it. Influenza places a substantial health burden on the United States every year, responsible for hundreds of thousands of hospitalizations and tens of thousands of deaths. The flu shot has been shown to prevent millions of illnesses each year (more than six million during the 2022-2023 season). And while it’s still possible to catch the flu after getting the flu shot, studies show that people are far less likely to be hospitalized or die when they’re vaccinated.
Another unexpected benefit of dropping the Yamagata strain from the seasonal vaccine? This will possibly make production of the flu vaccine faster, and enable manufacturers to make more vaccines, helping countries who have a flu vaccine shortage and potentially saving millions more lives.
After his grandmother’s dementia diagnosis, one man invented a snack to keep her healthy and hydrated.
Founder Lewis Hornby and his grandmother Pat, sampling Jelly Drops—an edible gummy containing water and life-saving electrolytes.
On a visit to his grandmother’s nursing home in 2016, college student Lewis Hornby made a shocking discovery: Dehydration is a common (and dangerous) problem among seniors—especially those that are diagnosed with dementia.
Hornby’s grandmother, Pat, had always had difficulty keeping up her water intake as she got older, a common issue with seniors. As we age, our body composition changes, and we naturally hold less water than younger adults or children, so it’s easier to become dehydrated quickly if those fluids aren’t replenished. What’s more, our thirst signals diminish naturally as we age as well—meaning our body is not as good as it once was in letting us know that we need to rehydrate. This often creates a perfect storm that commonly leads to dehydration. In Pat’s case, her dehydration was so severe she nearly died.
When Lewis Hornby visited his grandmother at her nursing home afterward, he learned that dehydration especially affects people with dementia, as they often don’t feel thirst cues at all, or may not recognize how to use cups correctly. But while dementia patients often don’t remember to drink water, it seemed to Hornby that they had less problem remembering to eat, particularly candy.

Hornby wanted to create a solution for elderly people who struggled keeping their fluid intake up. He spent the next eighteen months researching and designing a solution and securing funding for his project. In 2019, Hornby won a sizable grant from the Alzheimer’s Society, a UK-based care and research charity for people with dementia and their caregivers. Together, through the charity’s Accelerator Program, they created a bite-sized, sugar-free, edible jelly drop that looked and tasted like candy. The candy, called Jelly Drops, contained 95% water and electrolytes—important minerals that are often lost during dehydration. The final product launched in 2020—and was an immediate success. The drops were able to provide extra hydration to the elderly, as well as help keep dementia patients safe, since dehydration commonly leads to confusion, hospitalization, and sometimes even death.
Not only did Jelly Drops quickly become a favorite snack among dementia patients in the UK, but they were able to provide an additional boost of hydration to hospital workers during the pandemic. In NHS coronavirus hospital wards, patients infected with the virus were regularly given Jelly Drops to keep their fluid levels normal—and staff members snacked on them as well, since long shifts and personal protective equipment (PPE) they were required to wear often left them feeling parched.
In April 2022, Jelly Drops launched in the United States. The company continues to donate 1% of its profits to help fund Alzheimer’s research.

