Smartwatches can track COVID-19 symptoms, study finds

University of Michigan researchers found that new signals embedded in heart rate indicated when individuals were infected with COVID-19 and how ill they became.
If a COVID-19 infection develops, a wearable device may eventually be able to clue you in. A study at the University of Michigan found that a smartwatch can monitor how symptoms progress.
The study evaluated the effects of COVID-19 with various factors derived from heart-rate data. This method also could be employed to detect other diseases, such as influenza and the common cold, at home or when medical resources are limited, such as during a pandemic or in developing countries.
Tracking students and medical interns across the country, the University of Michigan researchers found that new signals embedded in heart rate indicated when individuals were infected with COVID-19 and how ill they became.
For instance, they discovered that individuals with COVID-19 experienced an increase in heart rate per step after the onset of their symptoms. Meanwhile, people who reported a cough as one of their COVID-19 symptoms had a much more elevated heart rate per step than those without a cough.
“We previously developed a variety of algorithms to analyze data from wearable devices. So, when the COVID-19 pandemic hit, it was only natural to apply some of these algorithms to see if we can get a better understanding of disease progression,” says Caleb Mayer, a doctoral student in mathematics at the University of Michigan and a co-first author of the study.
People may not internally sense COVID-19’s direct impact on the heart, but “heart rate is a vital sign that gives a picture of overall health," says Daniel Forger, a University of Michigan professor.
Millions of people are tracking their heart rate through wearable devices. This information is already generating a tremendous amount of data for researchers to analyze, says co-author Daniel Forger, professor of mathematics and research professor of computational medicine and bioinformatics at the University of Michigan.
“Heart rate is affected by many different physiological signals,” Forger explains. “For instance, if your lungs aren’t functioning properly, your heart may need to beat faster to meet metabolic demands. Your heart rate has a natural daily rhythm governed by internal biological clocks.” While people may not internally sense COVID-19’s direct impact on the heart, he adds that “heart rate is a vital sign that gives a picture of overall health.”
Among the total of 2,164 participants who enrolled in the student study, 72 undergraduate and graduate students contracted COVID-19, providing wearable data from 50 days before symptom onset to 14 days after. The researchers also analyzed this type of data for 43 medical interns from the Intern Health Study by the Michigan Neuroscience Institute and 29 individuals (who are not affiliated with the university) from the publicly available dataset.
Participants could wear the device on either wrist. They also documented their COVID-19 symptoms, such as fever, shortness of breath, cough, runny nose, vomiting, diarrhea, body aches, loss of taste, loss of smell, and sore throat.
Experts not involved in the study found the research to be productive. “This work is pioneering and reveals exciting new insights into the many important ways that we can derive clinically significant information about disease progression from consumer-grade wearable devices,” says Lisa A. Marsch, director of the Center for Technology and Behavioral Health and a professor in the Geisel School of Medicine at Dartmouth College. “Heart-rate data are among the highest-quality data that can be obtained via wearables.”
Beyond the heart, she adds, “Wearable devices are providing novel insights into individuals’ physiology and behavior in many health domains.” In particular, “this study beautifully illustrates how digital-health methodologies can markedly enhance our understanding of differences in individuals’ experience with disease and health.”
Previous studies had demonstrated that COVID-19 affects cardiovascular functions. Capitalizing on this knowledge, the University of Michigan endeavor took “a giant step forward,” says Gisele Oda, a researcher at the Institute of Biosciences at the University of Sao Paulo in Brazil and an expert in chronobiology—the science of biological rhythms. She commends the researchers for developing a complex algorithm that “could extract useful information beyond the established knowledge that heart rate increases and becomes more irregular in COVID patients.”
Wearable devices open the possibility of obtaining large-scale, long, continuous, and real-time heart-rate data on people performing everyday activities or while sleeping. “Importantly, the conceptual basis of this algorithm put circadian rhythms at the center stage,” Oda says, referring to the physical, mental, and behavioral changes that follow a 24-hour cycle. “What we knew before was often based on short-time heart rate measured at any time of day,” she adds, while noting that heart rate varies between day and night and also changes with activity.
However, without comparison to a control group of people having the common flu, it is difficult to determine if the heart-rate signals are unique to COVID-19 or also occur with other illnesses, says John Torous, an assistant professor of psychiatry at Harvard Medical School who has researched wearable devices. In addition, he points to recent data showing that many wearables, which work by beaming light through the skin, may be less accurate in people with darker skin due to variations in light absorption.
While the results sound interesting, they lack the level of conclusive evidence that would be needed to transform how physicians care for patients. “But it is a good step in learning more about what these wearables can tell us,” says Torous, who is also director of digital psychiatry at Beth Israel Deaconess Medical Center, a Harvard affiliate, in Boston. A follow-up step would entail replicating the results in a different pool of people to “help us realize the full value of this work.”
It is important to note that this research was conducted in university settings during the early phases of the pandemic, with remote learning in full swing amid strict isolation and quarantine mandates in effect. The findings demonstrate that physiological monitoring can be performed using consumer-grade wearable sensors, allowing research to continue without in-person contact, says Sung Won Choi, a professor of pediatrics at the University of Michigan who is principal investigator of the student study.
“The worldwide COVID-19 pandemic interrupted a lot of activities that relied on face-to-face interactions, including clinical research,” Choi says. “Mobile technology proved to be tremendously beneficial during that time, because it allowed us to collect detailed physiological data from research participants remotely over an entire semester.” In fact, the researchers did not have any in-person contact with the students involved in the study. “Everything was done virtually," Choi explains. "Importantly, their willingness to participate in research and share data during this historical time, combined with the capacity of secure cloud storage and novel mathematical analytics, enabled our research teams to identify unique patterns in heart-rate data associated with COVID-19.”
Paralyzed By Polio, This British Tea Broker Changed the Course Of Medical History Forever
Robin Cavendish in his special wheelchair with his son Jonathan in the 1960s.
In December 1958, on a vacation with his wife in Kenya, a 28-year-old British tea broker named Robin Cavendish became suddenly ill. Neither he nor his wife Diana knew it at the time, but Robin's illness would change the course of medical history forever.
Robin was rushed to a nearby hospital in Kenya where the medical staff delivered the crushing news: Robin had contracted polio, and the paralysis creeping up his body was almost certainly permanent. The doctors placed Robin on a ventilator through a tracheotomy in his neck, as the paralysis from his polio infection had rendered him unable to breathe on his own – and going off the average life expectancy at the time, they gave him only three months to live. Robin and Diana (who was pregnant at the time with their first child, Jonathan) flew back to England so he could be admitted to a hospital. They mentally prepared to wait out Robin's final days.
But Robin did something unexpected when he returned to the UK – just one of many things that would astonish doctors over the next several years: He survived. Diana gave birth to Jonathan in February 1959 and continued to visit Robin regularly in the hospital with the baby. Despite doctors warning that he would soon succumb to his illness, Robin kept living.
After a year in the hospital, Diana suggested something radical: She wanted Robin to leave the hospital and live at home in South Oxfordshire for as long as he possibly could, with her as his nurse. At the time, this suggestion was unheard of. People like Robin who depended on machinery to keep them breathing had only ever lived inside hospital walls, as the prevailing belief was that the machinery needed to keep them alive was too complicated for laypeople to operate. But Diana and Robin were up for the challenges – and the risks. Because his ventilator ran on electricity, if the house were to unexpectedly lose power, Diana would either need to restore power quickly or hand-pump air into his lungs to keep him alive.
Robin's wheelchair was not only the first of its kind; it became the model for the respiratory wheelchairs that people still use today.
In an interview as an adult, Jonathan Cavendish reflected on his parents' decision to live outside the hospital on a ventilator: "My father's mantra was quality of life," he explained. "He could have stayed in the hospital, but he didn't think that was as good of a life as he could manage. He would rather be two minutes away from death and living a full life."
After a few years of living at home, however, Robin became tired of being confined to his bed. He longed to sit outside, to visit friends, to travel – but had no way of doing so without his ventilator. So together with his friend Teddy Hall, a professor and engineer at Oxford University, the two collaborated in 1962 to create an entirely new invention: a battery-operated wheelchair prototype with a ventilator built in. With this, Robin could now venture outside the house – and soon the Cavendish family became famous for taking vacations. It was something that, by all accounts, had never been done before by someone who was ventilator-dependent. Robin and Hall also designed a van so that the wheelchair could be plugged in and powered during travel. Jonathan Cavendish later recalled a particular family vacation that nearly ended in disaster when the van broke down outside of Barcelona, Spain:
"My poor old uncle [plugged] my father's chair into the wrong socket," Cavendish later recalled, causing the electricity to short. "There was fire and smoke, and both the van and the chair ground to a halt." Johnathan, who was eight or nine at the time, his mother, and his uncle took turns hand-pumping Robin's ventilator by the roadside for the next thirty-six hours, waiting for Professor Hall to arrive in town and repair the van. Rather than being panicked, the Cavendishes managed to turn the vigil into a party. Townspeople came to greet them, bringing food and music, and a local priest even stopped by to give his blessing.
Robin had become a pioneer, showing the world that a person with severe disabilities could still have mobility, access, and a fuller quality of life than anyone had imagined. His mission, along with Hall's, then became gifting this independence to others like himself. Robin and Hall raised money – first from the Ernest Kleinwort Charitable Trust, and then from the British Department of Health – to fund more ventilator chairs, which were then manufactured by Hall's company, Littlemore Scientific Engineering, and given to fellow patients who wanted to live full lives at home. Robin and Hall used themselves as guinea pigs, testing out different models of the chairs and collaborating with scientists to create other devices for those with disabilities. One invention, called the Possum, allowed paraplegics to control things like the telephone and television set with just a nod of the head. Robin's wheelchair was not only the first of its kind; it became the model for the respiratory wheelchairs that people still use today.
Robin went on to enjoy a long and happy life with his family at their house in South Oxfordshire, surrounded by friends who would later attest to his "down-to-earth" personality, his sense of humor, and his "irresistible" charm. When he died peacefully at his home in 1994 at age 64, he was considered the world's oldest-living person who used a ventilator outside the hospital – breaking yet another barrier for what medical science thought was possible.
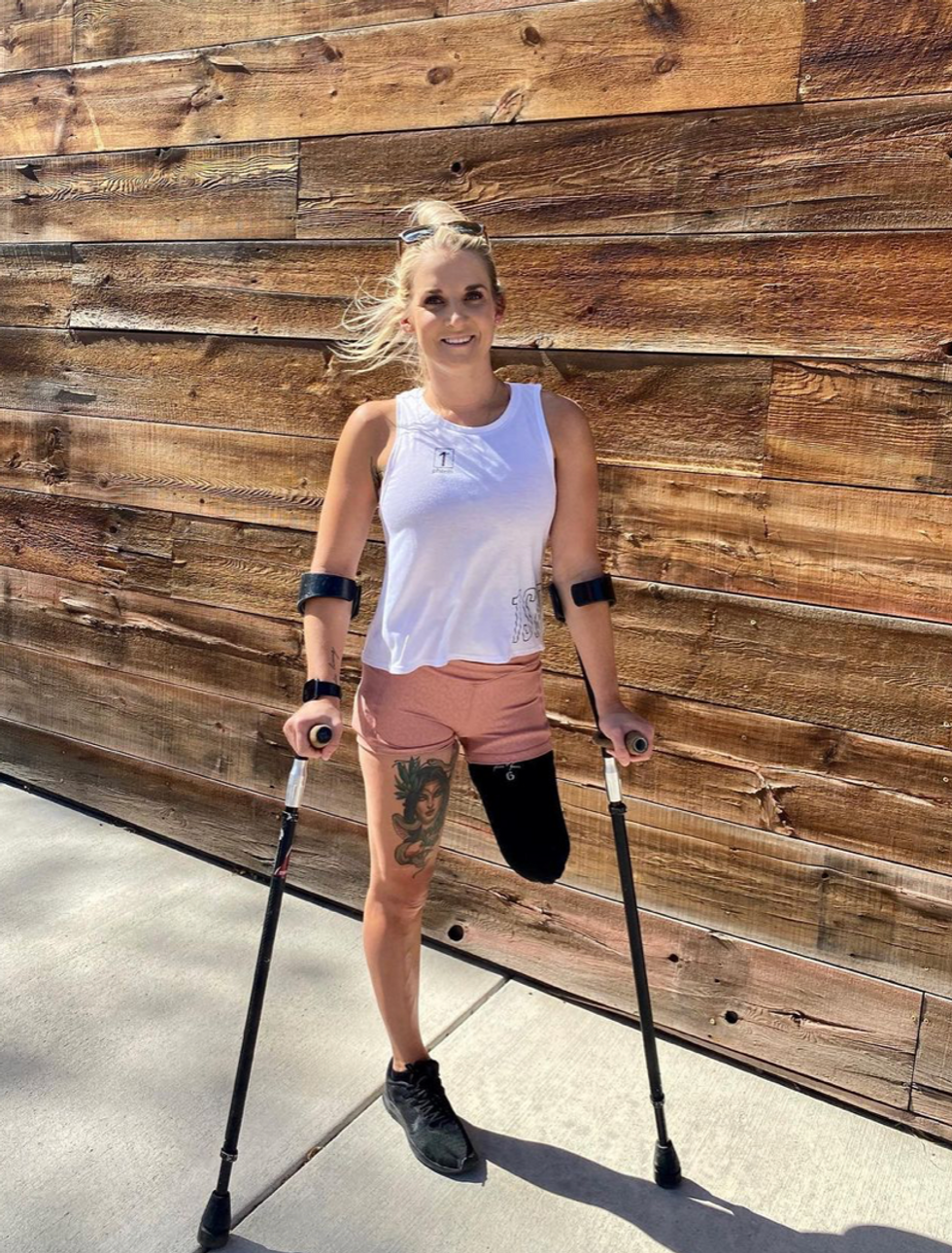
Kirstie Ennis, an Afghanistan veteran who survived a helicopter crash but lost a limb, pictured in May 2021 at Two Rivers Park in Colorado.
In June 2012, Kirstie Ennis was six months into her second deployment to Afghanistan and recently promoted to sergeant. The helicopter gunner and seven others were three hours into a routine mission of combat resupplies and troop transport when their CH-53D helicopter went down hard.
Miraculously, all eight people onboard survived, but Ennis' injuries were many and severe. She had a torn rotator cuff, torn labrum, crushed cervical discs, facial fractures, deep lacerations and traumatic brain injury. Despite a severely fractured ankle, doctors managed to save her foot, for a while at least.
In November 2015, after three years of constant pain and too many surgeries to count, Ennis relented. She elected to undergo a lower leg amputation but only after she completed the 1,000-mile, 72-day Walking with the Wounded journey across the UK.
On Veteran's Day of that year, on the other side of the country, orthopedic surgeon Cato Laurencin announced a moonshot challenge he was setting out to achieve on behalf of wounded warriors like Ennis: the Hartford Engineering A Limb (HEAL) Project.
Laurencin, who is a University of Connecticut professor of chemical, materials and biomedical engineering, teamed up with experts in tissue bioengineering and regenerative medicine from Harvard, Columbia, UC Irvine and SASTRA University in India. Laurencin and his colleagues at the Connecticut Convergence Institute for Translation in Regenerative Engineering made a bold commitment to regenerate an entire limb within 15 years – by the year 2030.

Dr. Cato Laurencin pictured in his office at UConn.
Photo Credit: UConn
Regenerative Engineering -- A Whole New Field
Limb regeneration in humans has been a medical and scientific fascination for decades, with little to show for the effort. However, Laurencin believes that if we are to reach the next level of 21st century medical advances, this puzzle must be solved.
An estimated 185,000 people undergo upper or lower limb amputation every year. Despite the significant advances in electromechanical prosthetics, these individuals still lack the ability to perform complex functions such as sensation for tactile input, normal gait and movement feedback. As far as Laurencin is concerned, the only clinical answer that makes sense is to regenerate a whole functional limb.
Laurencin feels other regeneration efforts were hampered by their siloed research methods with chemists, surgeons, engineers all working separately. Success, he argues, requires a paradigm shift to a trans-disciplinary approach that brings together cutting-edge technologies from disparate fields such as biology, material sciences, physical, chemical and engineering sciences.
As the only surgeon ever inducted into the academies of Science, Medicine and Innovation, Laurencin is uniquely suited for the challenge. He is regarded as the founder of Regenerative Engineering, defined as the convergence of advanced materials sciences, stem cell sciences, physics, developmental biology and clinical translation for the regeneration of complex tissues and organ systems.
But none of this is achievable without early clinician participation across scientific fields to develop new technologies and a deeper understanding of how to harness the body's innate regenerative capabilities. "When I perform a surgical procedure or something is torn or needs to be repaired, I count on the body being involved in regenerating tissue," he says. "So, understanding how the body works to regenerate itself and harnessing that ability is an important factor for the regeneration process."
The Birth of the Vision
Laurencin's passion for regeneration began when he was a sports medicine fellow at Cornell University Medical Center in the early 1990s. There he saw a significant number of injuries to the anterior cruciate ligament (ACL), the major ligament that stabilizes the knee. He believed he could develop a better way to address those injuries using biomaterials to regenerate the ligament. He sketched out a preliminary drawing on a napkin one night over dinner. He has spent the next 30 years regenerating tissues, including the patented L-C ligament.
As chair of Orthopaedic Surgery at the University of Virginia during the peak of the wars in Iraq and Afghanistan, Laurencin treated military personnel who survived because of improved helmets, body armor and battlefield medicine but were left with more devastating injuries, including traumatic brain injuries and limb loss.
"I was so honored to care for them and I so admired their steadfast courage that I became determined to do something big for them," says Laurencin.
When he tells people about his plans to regrow a limb, he gets a lot of eye rolls, which he finds amusing but not discouraging. Growing bone cells was relatively new when he was first focused on regenerating bone in 1987 at MIT; in 2007 he was well on his way to regenerating ligaments at UVA when many still doubted that ligaments could even be reconstructed. He and his team have already regenerated torn rotator cuff tendons and ACL ligaments using a nano-textured fabric seeded with stem cells.
Even as a finalist for the $4 million NIH Pioneer Award for high-risk/high-reward research, he faced a skeptical scientific audience in 2014. "They said, 'Well what do you plan to do?' I said 'I plan to regenerate a whole limb in people.' There was a lot of incredulousness. They stared at me and asked a lot of questions. About three days later, I received probably the best score I've ever gotten on an NIH grant."
In the Thick of the Science
Humans are born with regenerative abilities--two-year-olds have regrown fingertips--but lose that ability with age. Salamanders are the only vertebrates that can regenerate lost body parts as adults; axolotl, the rare Mexican salamander, can grow extra limbs.
The axolotl is important as a model organism because it is a four-footed vertebrate with a similar body plan to humans. Mapping the axolotl genome in 2018 enhanced scientists' genetic understanding of their evolution, development, and regeneration. Being easy to breed in captivity allowed the HEAL team to closely study these amphibians and discover a new cell type they believe may shed light on how to mimic the process in humans.
"Whenever limb regeneration takes place in the salamander, there is a huge amount of something called heparan sulfate around that area," explains Laurencin. "We thought, 'What if this heparan sulfate is the key ingredient to allowing regeneration to take place?' We found these groups of cells that were interspersed in tissues during the time of regeneration that seemed to have connections to each other that expressed this heparan sulfate."
Called GRID (Groups that are Regenerative, Interspersed and Dendritic), these cells were also recently discovered in mice. While GRID cells don't regenerate as well in mice as in salamanders, finding them in mammals was significant.
"If they're found in mice. we might be able to find these in humans in some form," Laurencin says. "We think maybe it will help us figure out regeneration or we can create cells that mimic what grid cells do and create an artificial grid cell."
What Comes Next?
Laurencin and his team have individually engineered and made every single tissue in the lower limb, including bone, cartilage, ligament, skin, nerve, blood vessels. Regenerating joints and joint tissue is the next big mile marker, which Laurencin sees as essential to regenerating a limb that functions and performs in the way he envisions.
"Using stem cells and amnion tissue, we can regenerate joints that are damaged, and have severe arthritis," he says. "We're making progress on all fronts, and making discoveries we believe are going to be helping people along the way."
That focus and advancement is vital to Ennis. After laboring over the decision to have her leg amputated below the knee, she contracted MRSA two weeks post-surgery. In less than a month, she went from a below-the-knee-amputee to a through-the-knee amputee to an above-the-knee amputee.
"A below-the-knee amputation is night-and-day from above-the-knee," she said. "You have to relearn everything. You're basically a toddler."
Kirstie Ennis pictured in July 2020.
Photo Credit: Ennis' Instagram
The clock is ticking on the timeline Laurencin set for himself. Nine years might seem like forever if you're doing time but it might appear fleeting when you're trying to create something that's never been done before. But Laurencin isn't worried. He's convinced time is on his side.
"Every week, I receive an email or a call from someone, maybe a mother whose child has lost a finger or I'm in communication with a disabled American veteran who wants to know how the progress is going. That energizes me to continue to work hard to try to create these sorts of solutions because we're talking about people and their lives."
He devotes about 60 hours a week to the project and the roughly 100 students, faculty and staff who make up the HEAL team at the Convergence Institute seem acutely aware of what's at stake and appear equally dedicated.
"We're in the thick of the science in terms of making this happen," says Laurencin. "We've moved from making the impossible possible to making the possible a reality. That's what science is all about."

