A new injection is helping stave off RSV this season
The FDA approved a single-dose, long-acting injection to protect babies and toddlers from RSV over the fall and winter.
In November 2021, Mickayla Wininger’s then one-month-old son, Malcolm, endured a terrifying bout with RSV, the respiratory syncytial (sin-SISH-uhl) virus—a common ailment that affects all age groups. Most people recover from mild, cold-like symptoms in a week or two, but RSV can be life-threatening in others, particularly infants.
Wininger, who lives in southern Illinois, was dressing Malcolm for bed when she noticed what seemed to be a minor irregularity with this breathing. She and her fiancé, Gavin McCullough, planned to take him to the hospital the next day. The matter became urgent when, in the morning, the boy’s breathing appeared to have stopped.
After they dialed 911, Malcolm started breathing again, but he ended up being hospitalized three times for RSV and defects in his heart. Eventually, he recovered fully from RSV, but “it was our worst nightmare coming to life,” Wininger recalled.
It’s a scenario that the federal government is taking steps to prevent. In July, the Food and Drug Administration approved a single-dose, long-acting injection to protect babies and toddlers. The injection, called Beyfortus, or nirsevimab, became available this October. It reduces the incidence of RSV in pre-term babies and other infants for their first RSV season. Children at highest risk for severe RSV are those who were born prematurely and have either chronic lung disease of prematurity or congenital heart disease. In those cases, RSV can progress to lower respiratory tract diseases such as pneumonia and bronchiolitis, or swelling of the lung’s small airway passages.
Each year, RSV is responsible for 2.1 million outpatient visits among children younger than five-years-old, 58,000 to 80,000 hospitalizations in this age group, and between 100 and 300 deaths, according to the Centers for Disease Control and Prevention. Transmitted through close contact with an infected person, the virus circulates on a seasonal basis in most regions of the country, typically emerging in the fall and peaking in the winter.
In August, however, the CDC issued a health advisory on a late-summer surge in severe cases of RSV among young children in Florida and Georgia. The agency predicts "increased RSV activity spreading north and west over the following two to three months.”
Infants are generally more susceptible to RSV than older people because their airways are very small, and their mechanisms to clear these passages are underdeveloped. RSV also causes mucus production and inflammation, which is more of a problem when the airway is smaller, said Jennifer Duchon, an associate professor of newborn medicine and pediatrics in the Icahn School of Medicine at Mount Sinai in New York.
In 2021 and 2022, RSV cases spiked, sending many to emergency departments. “RSV can cause serious disease in infants and some children and results in a large number of emergency department and physician office visits each year,” John Farley, director of the Office of Infectious Diseases in the FDA’s Center for Drug Evaluation and Research, said in a news release announcing the approval of the RSV drug. The decision “addresses the great need for products to help reduce the impact of RSV disease on children, families and the health care system.”
Sean O’Leary, chair of the committee on infectious diseases for the American Academy of Pediatrics, says that “we’ve never had a product like this for routine use in children, so this is very exciting news.” It is recommended for all kids under eight months old for their first RSV season. “I would encourage nirsevimab for all eligible children when it becomes available,” O’Leary said.
For those children at elevated risk of severe RSV and between the ages of 8 and 19 months, the CDC recommends one dose in their second RSV season.
The drug will be “really helpful to keep babies healthy and out of the hospital,” said O’Leary, a professor of pediatrics at the University of Colorado Anschutz Medical Campus/Children’s Hospital Colorado in Denver.
An antiviral drug called Synagis (palivizumab) has been an option to prevent serious RSV illness in high-risk infants since it was approved by the FDA in 1998. The injection must be given monthly during RSV season. However, its use is limited to “certain children considered at high risk for complications, does not help cure or treat children already suffering from serious RSV disease, and cannot prevent RSV infection,” according to the National Foundation for Infectious Diseases.
Until the approval this summer of the new monoclonal antibody, nirsevimab, there wasn’t a reliable method to prevent infection in most healthy infants.
Both nirsevimab and palivizumab are monoclonal antibodies that act against RSV. Monoclonal antibodies are lab-made proteins that mimic the immune system’s ability to fight off harmful pathogens such as viruses. A single intramuscular injection of nirsevimab preceding or during RSV season may provide protection.
The strategy with the new monoclonal antibody is “to extend protection to healthy infants who nonetheless are at risk because of their age, as well as infants with additional medical risk factors,” said Philippa Gordon, a pediatrician and infectious disease specialist in Brooklyn, New York, and medical adviser to Park Slope Parents, an online community support group.
No specific preventive measure is needed for older and healthier kids because they will develop active immunity, which is more durable. Meanwhile, older adults, who are also vulnerable to RSV, can receive one of two new vaccines. So can pregnant women, who pass on immunity to the fetus, Gordon said.
Until the approval this summer of the new monoclonal antibody, nirsevimab, there wasn’t a reliable method to prevent infection in most healthy infants, “nor is there any treatment other than giving oxygen or supportive care,” said Stanley Spinner, chief medical officer and vice president of Texas Children’s Pediatrics and Texas Children’s Urgent Care.
As with any virus, washing hands frequently and keeping infants and children away from sick people are the best defenses, Duchon said. This approach isn’t foolproof because viruses can run rampant in daycare centers, schools and parents’ workplaces, she added.
Mickayla Wininger, Malcolm’s mother, insists that family and friends wear masks, wash their hands and use hand sanitizer when they’re around her daughter and two sons. She doesn’t allow them to kiss or touch the children. Some people take it personally, but she would rather be safe than sorry.
Wininger recalls the severe anxiety caused by Malcolm's ordeal with RSV. After returning with her infant from his hospital stays, she was terrified to go to sleep. “My fiancé and I would trade shifts, so that someone was watching over our son 24 hours a day,” she said. “I was doing a night shift, so I would take caffeine pills to try and keep myself awake and would end up crashing early hours in the morning and wake up frantically thinking something happened to my son.”
Two years later, her anxiety has become more manageable, and Malcolm is doing well. “He is thriving now,” Wininger said. He recently had his second birthday and "is just the spunkiest boy you will ever meet. He looked death straight in the eyes and fought to be here today.”
Many children use makeup and body products such as glitter, face paint and lip gloss. Scientists are pointing to the harmful chemicals in some of these products, and advocates are looking to outlaw them.
When Erika Schreder’s 14-year-old daughter, who is Black, had her curly hair braided at a Seattle-area salon two or three times recently, the hairdresser applied a styling gel to seal the tresses in place.
Schreder and her daughter had been trying to avoid harmful chemicals, so they were shocked to later learn that this particular gel had the highest level of formaldehyde of any product tested by the Washington State Departments of Ecology and Health. In January 2023, the agencies released a report that uncovered high levels of formaldehyde in certain hair products, creams and lotions marketed to or used by people of color. When Schreder saw the report, she mentioned it to her daughter, who told her the name of the gel smoothed on her hair.
“It was really upsetting,” said Schreder, science director at Toxic-Free Future, a Seattle-based nonprofit environmental health research and advocacy organization. “Learning that this product used on my daughter’s hair contained cancer-causing formaldehyde made me even more committed to advocating for our state to ban toxic ingredients in cosmetics and personal care products.”
In 2013, Toxic-Free Future launched Mind the Store to challenge the nation’s largest retailers in adopting comprehensive policies that eliminate toxic chemicals in their personal care products and packaging, and develop safer alternatives.
Now, more efforts are underway to expose and mitigate the harm in cosmetics, hair care and other products that children apply on their faces, heads, nails and other body parts. Advocates hope to raise awareness among parents while prompting manufacturers and salon professionals to adopt safer alternatives.
A recent study by researchers at Columbia University Mailman School of Public Health and Earthjustice, a San Francisco-based nonprofit public interest environmental law organization, revealed that most children in the United States use makeup and body products that may contain carcinogens and other toxic chemicals. In January, the results were published in the International Journal of Environmental Research and Public Health. Based on more than 200 surveys, 70 percent of parents in the study reported that their children 12 or younger have used makeup and body products marketed to youth — for instance, glitter, face paint and lip gloss.
Childhood exposure to harmful makeup and body product ingredients can also be considered an environmental justice issue, as communities of color may be more likely to use these products.
“We are concerned about exposure to chemicals that may be found in cosmetics and body products, including those that are marketed toward children,” said the study’s senior author, Julie Herbstman, a professor and director of the Columbia Center for Children's Environmental Health. The goal of the survey was to try to understand how much kids are using cosmetic and body products and when, how and why they are using them.
“There is widespread use of children’s cosmetic and body products, and kids are using them principally to play,” Herbstman said. “That’s really quite different than how adults use cosmetic and body products.” Even with products that are specifically designed for children, “there’s no regulation that ensures that these products are safe for kids.” Also, she said, some children are using adult products — and they may do so in inadvisable ways, such as ingesting lipstick or applying it to other areas of the face.
Earlier research demonstrated that beauty and personal care products manufactured for children and adults frequently contain toxic chemicals, such as lead, asbestos, PFAS, phthalates and formaldehyde. Heavy metals and other toxic chemicals in children’s makeup and body products are particularly harmful to infants and youth, who are growing rapidly and whose bodies are less efficient at metabolizing these chemicals. Whether these chemicals are added intentionally or are present as contaminants, they have been associated with cancer, neurodevelopmental harm, and other serious and irreversible health effects, the Columbia University and Earthjustice researchers noted.
“Even when concentrations of individual chemicals are low in products, the potential for interactive effects from multiple toxicants is important to take into consideration,” the authors wrote in the journal article. “Allergic reactions, such as contact dermatitis, are some of the most frequently cited negative health outcomes associated with the use of cosmetics.”

Children’s small body side, rapid growth rate and immature immune systems are biologically more prone to the effects of toxicants than adults.
Adobe Stock
In addition to children’s rapid growth rate, the study also reported that their small body size, developing tissues and organs, and immature immune systems are biologically more prone to the effects of toxicants than adults. Meanwhile, the study noted, “childhood exposure to harmful makeup and body product ingredients can also be considered an environmental justice issue, as communities of color may be more likely to use these products.”
Although adults are the typical users of cosmetics, similar items are heavily marketed to youth with attention-grabbing features such as bright colors, animals and cartoon characters, according to the study. Beyond conventional makeup such as eyeshadow and lipstick, children may apply face paint, body glitter, nail polish, hair gel and fragrances. They also may frequent social media platforms on which these products are increasingly being promoted.
Products for both children and adults are currently regulated by the U.S. Food and Drug Administration under the Federal Food, Drug, and Cosmetic Act of 1938. Also, the Fair Packaging and Labeling Act of 1967 directs the Federal Trade Commission and the FDA “to issue regulations requiring that all ‘consumer commodities’ be labeled to disclose net contents, identity of commodity, and name and place of business of the product's manufacturer, packer, or distributor.” As the Columbia University and Earthjustice authors pointed out, though, “current safety regulations have been widely criticized as inadequate.”
The Personal Care Products Council in Washington, D.C., “fundamentally disagrees with the premise that companies put toxic chemicals in products produced for children,” industry spokeswoman Lisa Powers said in an email. Founded in 1894, the national trade association represents 600 member companies that manufacture, distribute and supply most personal care products marketed in the United States.
No category of consumer products is subject to less government oversight than cosmetics and other personal care products. -- Environmental Working Group.
“Science and safety are the cornerstones of our industry,” Powers stated. For more than a decade, she wrote, “the [Council] and our member companies worked diligently with a bipartisan group of congressional leaders and a diverse group of stakeholders to enhance the effectiveness of the FDA regulatory authority and to provide the safety reassurances that consumers expect and deserve.”
Powers added that the “industry employs and consults thousands of scientific and medical experts” who study the impacts of cosmetics and personal care products and the ingredients used in them. The Council also maintains a comprehensive database where consumers can look up science and safety information on the thousands of ingredients in sunscreens, toothpaste, shampoo, moisturizer, makeup, fragrances and other products.
However, the Environmental Working Group, which empowers consumers with breakthrough research to make informed choices about healthy living, believes the regulations are still not robust enough. “No category of consumer products is subject to less government oversight than cosmetics and other personal care products,” states the organization’s website. “Although many of the chemicals and contaminants in cosmetics and personal care products likely pose little risk, exposure to some has been linked to serious health problems, including cancer.”
The group, which operates the Skin Deep Database noted that “since 2009, 595 cosmetics manufacturers have reported using 88 chemicals, in more than 73,000 products, that have been linked to cancer, birth defects or reproductive harm.”
But change, for both adults and kids, is on the horizon. The Modernization of Cosmetics Regulation Act of 2022 significantly expanded the FDA’s authority to regulate cosmetics. In May 2023, Washington state adopted a law regulating cosmetics and personal care products. The Toxic-Free Cosmetics Act (HB 1047) bans chemicals in beauty and personal care products, such as PFAS, lead, mercury, phthalates and formaldehyde-releasing agents. These bans take effect in 2025, except for formaldehyde releasers, which have a phased-in approach starting in 2026.
Industry and advocates view this as a positive development. Powers, the spokesperson, praised “the long-awaited” Modernization Cosmetics Regulation Act of 2022, which she said, “advances product safety and innovation.” Jen Lee, chief impact officer at Beautycoutner, a company that sells personal care products, also welcomes the change. “We were proud to support the Washington Toxic-Free Cosmetics Act (HB 1047) by mobilizing our community of Brand Advocates who reside in Washington State,” Lee said. “Together, they made their voices heard by sending over 1,000 emails to their state legislators urging them to support and pass the bill.”
Laurie Valeriano, executive director of Toxic-Free Future, praised the upcoming Washington state law as “a huge win for public health and the environment that will have impacts that ripple across the nation.” She added that “companies won’t make special products for Washington state.” Instead, “they will reformulate and make products safer for everyone” — adults and children.
You shouldn’t have to be a toxicologist to shop for shampoo. -- Washington State Rep. Sharlett Mena
The new legislation will require Washington state agencies to assess the hazards of chemicals used in products that can impact vulnerable populations, while providing support for small businesses and independent cosmetologists to transition to safer products.
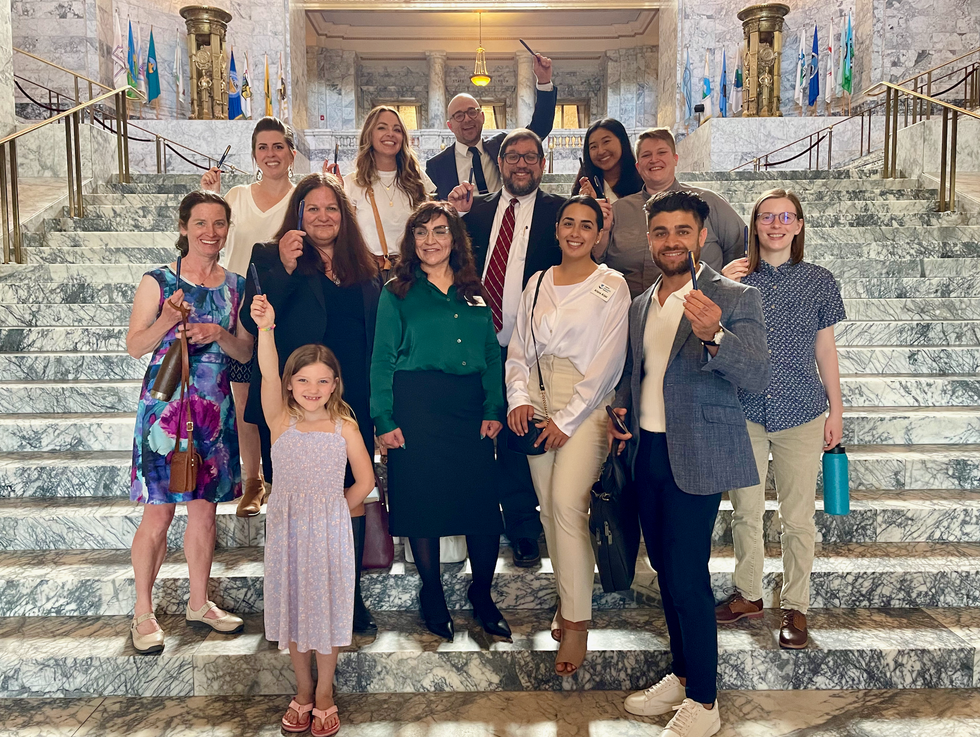
The Toxic-Free Future team lauds the Cosmetics Act, signed in May 2023.
Courtesy Toxic-Free Future
“When we go to a store, we assume the products on the shelf are safe, but this isn’t always true,” said Washington State Rep. Sharlett Mena, a Democrat serving in the 29th Legislative District (Tacoma), who sponsored the law. “I introduced this bill (HB 1047) because currently, the burden is on the consumer to navigate labels and find safe alternatives. You shouldn’t have to be a toxicologist to shop for shampoo.”
The new law aims to protect people of all ages, but especially youth. “Children are more susceptible to the impacts of toxic chemicals because their bodies are still developing,” Mena said. “Lead, for example, is significantly more hazardous to children than adults. Also, since children, unlike adults, tend to put things in their mouths all the time, they are more exposed to harmful chemicals in personal care and other products.”
Cosmetologists and hair professionals are taking notice. “Safety should be the practitioner’s number one concern” in using products on small children, said Anwar Saleem, a hair stylist, instructor and former salon owner in Washington, D.C., who is chairman of the D.C. Board of Barbering and Cosmetology and president of the National Interstate Council of State Boards of Cosmetology. “There are so many products on the market that it can be confusing.”
Hair products designed and labeled for children's use often have milder formulations, but “every child is unique, and what works for one may not work for another,” Saleem said. He recommends doing a patch test, in which the stylist or cosmetologist dabs the product on a small, inconspicuous area of the scalp or skin and waits anywhere from an hour to a day to check for irritation before continuing to serve the client. “Performing a patch test, observing children's reactions to a product and adequately adjusting are essential.”
Saleem seeks products that are free from harsh chemicals such as sulfates, phthalates and parabens, noting that these ingredients can be irritating and drying to the hair and scalp. If a child has sensitive skin or allergies, Saleem opts for hypoallergenic products.
We also need to ensure that less toxic alternatives are available and accessible to all consumers. It’s often under-resourced, low-income populations who suffer the burden of environmental exposures and do not have access or cannot afford these safer alternatives. -- Lesliam Quirós-Alcalá.
Lesliam Quirós-Alcalá, an assistant professor in the department of environmental health and engineering at the Johns Hopkins Bloomberg School of Public Health, said current regulatory loopholes on product labeling still allow manufacturers to advertise their cosmetics and personal care products as “gentle” and “natural.” However, she said, those terms may be misleading as they don’t necessarily mean the contents are less toxic or harmful to consumers.
“We also need to ensure that less toxic alternatives are available and accessible to all consumers,” Quirós-Alcalá said, “as often alternatives considered to be less toxic come with a hefty price tag.” As a result, “it’s often under-resourced, low-income populations who suffer the burden of environmental exposures and do not have access or cannot afford these safer alternatives.”
To advocate for safer alternatives, Quirós-Alcalá suggests that parents turn to consumer groups involved in publicizing the harms of personal care products. The Campaign for Safe Cosmetics is a program of Breast Cancer Prevention Partners, a national science-based advocacy organization aiming to prevent the disease by eliminating related environmental exposures. Other resources that inform users about unsafe ingredients include the mobile apps Clearya and Think Dirty.
“Children are not little adults, so it’s important to increase parent and consumer awareness to minimize their exposures to toxic chemicals in everyday products,” Quirós-Alcalá said. “Becoming smarter, more knowledgeable consumers is the first step to protecting your family from potentially harmful and toxic ingredients in consumer products.”
FDA, researchers work to make clinical trials more diverse
The U.S. population is becoming more diverse, but clinical trials don't reflect that, experts say. Some are focusing on recruiting minorities to participate in research.
Nestled in a predominately Hispanic neighborhood, a new mural outside Guadalupe Centers Middle School in Kansas City, Missouri imparts a powerful message: “Clinical Research Needs Representation.” The colorful portraits painted above those words feature four cancer survivors of different racial and ethnic backgrounds. Two individuals identify as Hispanic, one as African American and another as Native American.
One of the patients depicted in the mural is Kim Jones, a 51-year-old African American breast cancer survivor since 2012. She advocated for an African American friend who participated in several clinical trials for ovarian cancer. Her friend was diagnosed in an advanced stage at age 26 but lived nine more years, thanks to the trials testing new therapeutics. “They are definitely giving people a longer, extended life and a better quality of life,” said Jones, who owns a nail salon. And that’s the message the mural aims to send to the community: Clinical trials need diverse participants.
While racial and ethnic minority groups represent almost half of the U.S. population, the lack of diversity in clinical trials poses serious challenges. Limited awareness and access impede equitable representation, which is necessary to prove the safety and effectiveness of medical interventions across different groups.
A Yale University study on clinical trial diversity published last year in BMJ Medicine found that while 81 percent of trials testing the new cancer drugs approved by the U.S. Food and Drug Administration between 2012 and 2017 included women, only 23 percent included older adults and 5 percent fairly included racial and ethnic minorities. “It’s both a public health and social justice issue,” said Jennifer E. Miller, an associate professor of medicine at Yale School of Medicine. “We need to know how medicines and vaccines work for all clinically distinct groups, not just healthy young White males.” A recent JAMA Oncology editorial stresses out the need for legislation that would require diversity action plans for certain types of trials.
Ensuring meaningful representation of racial and ethnic minorities in clinical trials for regulated medical products is fundamental to public health.--FDA Commissioner Robert M. Califf.
But change is on the horizon. Last April, the FDA issued a new draft guidance encouraging industry to find ways to revamp recruitment into clinical trials. The announcement, which expanded on previous efforts, called for including more participants from underrepresented racial and ethnic segments of the population.
“The U.S. population has become increasingly diverse, and ensuring meaningful representation of racial and ethnic minorities in clinical trials for regulated medical products is fundamental to public health,” FDA commissioner Robert M. Califf, a physician, said in a statement. “Going forward, achieving greater diversity will be a key focus throughout the FDA to facilitate the development of better treatments and better ways to fight diseases that often disproportionately impact diverse communities. This guidance also further demonstrates how we support the Administration’s Cancer Moonshot goal of addressing inequities in cancer care, helping to ensure that every community in America has access to cutting-edge cancer diagnostics, therapeutics and clinical trials.”
Lola Fashoyin-Aje, associate director for Science and Policy to Address Disparities in the Oncology Center of Excellence at the FDA, said that the agency “has long held the view that clinical trial participants should reflect the clinical and demographic characteristics of the patients who will ultimately receive the drug once approved.” However, “numerous studies over many decades” have measured the extent of underrepresentation. One FDA analysis found that the proportion of White patients enrolled in U.S. clinical trials (88 percent) is much higher than their numbers in country's population. Meanwhile, the enrollment of African American and Native Hawaiian/American Indian and Alaskan Native patients is below their national numbers.
The FDA’s guidance is accelerating researchers’ efforts to be more inclusive of diverse groups in clinical trials, said Joyce Sackey, a clinical professor of medicine and associate dean at Stanford School of Medicine. Underrepresentation is “a huge issue,” she noted. Sackey is focusing on this in her role as the inaugural chief equity, diversity and inclusion officer at Stanford Medicine, which encompasses the medical school and two hospitals.
Until the early 1990s, Sackey pointed out, clinical trials were based on research that mainly included men, as investigators were concerned that women could become pregnant, which would affect the results. This has led to some unfortunate consequences, such as indications and dosages for drugs that cause more side effects in women due to biological differences. “We’ve made some progress in including women, but we have a long way to go in including people of different ethnic and racial groups,” she said.

A new mural outside Guadalupe Centers Middle School in Kansas City, Missouri, advocates for increasing diversity in clinical trials. Kim Jones, 51-year-old African American breast cancer survivor, is second on the left.
Artwork by Vania Soto. Photo by Megan Peters.
Among racial and ethnic minorities, distrust of clinical trials is deeply rooted in a history of medical racism. A prime example is the Tuskegee Study, a syphilis research experiment that started in 1932 and spanned 40 years, involving hundreds of Black men with low incomes without their informed consent. They were lured with inducements of free meals, health care and burial stipends to participate in the study undertaken by the U.S. Public Health Service and the Tuskegee Institute in Alabama.
By 1947, scientists had figured out that they could provide penicillin to help patients with syphilis, but leaders of the Tuskegee research failed to offer penicillin to their participants throughout the rest of the study, which lasted until 1972.
Opeyemi Olabisi, an assistant professor of medicine at Duke University Medical Center, aims to increase the participation of African Americans in clinical research. As a nephrologist and researcher, he is the principal investigator of a clinical trial focusing on the high rate of kidney disease fueled by two genetic variants of the apolipoprotein L1 (APOL1) gene in people of recent African ancestry. Individuals of this background are four times more likely to develop kidney failure than European Americans, with these two variants accounting for much of the excess risk, Olabisi noted.
The trial is part of an initiative, CARE and JUSTICE for APOL1-Mediated Kidney Disease, through which Olabisi hopes to diversify study participants. “We seek ways to engage African Americans by meeting folks in the community, providing accessible information and addressing structural hindrances that prevent them from participating in clinical trials,” Olabisi said. The researchers go to churches and community organizations to enroll people who do not visit academic medical centers, which typically lead clinical trials. Since last fall, the initiative has screened more than 250 African Americans in North Carolina for the genetic variants, he said.
Other key efforts are underway. “Breaking down barriers, including addressing access, awareness, discrimination and racism, and workforce diversity, are pivotal to increasing clinical trial participation in racial and ethnic minority groups,” said Joshua J. Joseph, assistant professor of medicine at the Ohio State University Wexner Medical Center. Along with the university’s colleges of medicine and nursing, researchers at the medical center partnered with the African American Male Wellness Agency, Genentech and Pfizer to host webinars soliciting solutions from almost 450 community members, civic representatives, health care providers, government organizations and biotechnology professionals in 25 states and five countries.
Their findings, published in February in the journal PLOS One, suggested that including incentives or compensation as part of the research budget at the institutional level may help resolve some issues that hinder racial and ethnic minorities from participating in clinical trials. Compared to other groups, more Blacks and Hispanics have jobs in service, production and transportation, the authors note. It can be difficult to get paid leave in these sectors, so employees often can’t join clinical trials during regular business hours. If more leaders of trials offer money for participating, that could make a difference.
Obstacles include geographic access, language and other communications issues, limited awareness of research options, cost and lack of trust.
Christopher Corsico, senior vice president of development at GSK, formerly GlaxoSmithKline, said the pharmaceutical company conducted a 17-year retrospective study on U.S. clinical trial diversity. “We are using epidemiology and patients most impacted by a particular disease as the foundation for all our enrollment guidance, including study diversity plans,” Corsico said. “We are also sharing our results and ideas across the pharmaceutical industry.”
Judy Sewards, vice president and head of clinical trial experience at Pfizer’s headquarters in New York, said the company has committed to achieving racially and ethnically diverse participation at or above U.S. census or disease prevalence levels (as appropriate) in all trials. “Today, barriers to clinical trial participation persist,” Sewards said. She noted that these obstacles include geographic access, language and other communications issues, limited awareness of research options, cost and lack of trust. “Addressing these challenges takes a village. All stakeholders must come together and work collaboratively to increase diversity in clinical trials.”
It takes a village indeed. Hope Krebill, executive director of the Masonic Cancer Alliance, the outreach network of the University of Kansas Cancer Center in Kansas City, which commissioned the mural, understood that well. So her team actively worked with their metaphorical “village.” “We partnered with the community to understand their concerns, knowledge and attitudes toward clinical trials and research,” said Krebill. “With that information, we created a clinical trials video and a social media campaign, and finally, the mural to encourage people to consider clinical trials as an option for care.”
Besides its encouraging imagery, the mural will also be informational. It will include a QR code that viewers can scan to find relevant clinical trials in their location, said Vania Soto, a Mexican artist who completed the rendition in late February. “I’m so honored to paint people that are survivors and are living proof that clinical trials worked for them,” she said.
Jones, the cancer survivor depicted in the mural, hopes the image will prompt people to feel more open to partaking in clinical trials. “Hopefully, it will encourage people to inquire about what they can do — how they can participate,” she said.


