Alzheimer’s prevention may be less about new drugs, more about income, zip code and education

(Left to right) Vickie Naylor, Bernadine Clay, and Donna Maxey read a memory prompt as they take part in the Sharing History through Active Reminiscence and Photo-Imagery (SHARP) study, September 20, 2017.
That your risk of Alzheimer’s disease depends on your salary, what you ate as a child, or the block where you live may seem implausible. But researchers are discovering that social determinants of health (SDOH) play an outsized role in Alzheimer’s disease and related dementias, possibly more than age, and new strategies are emerging for how to address these factors.
At the 2022 Alzheimer’s Association International Conference, a series of presentations offered evidence that a string of socioeconomic factors—such as employment status, social support networks, education and home ownership—significantly affected dementia risk, even when adjusting data for genetic risk. What’s more, memory declined more rapidly in people who earned lower wages and slower in people who had parents of higher socioeconomic status.
In 2020, a first-of-its kind study in JAMA linked Alzheimer’s incidence to “neighborhood disadvantage,” which is based on SDOH indicators. Through autopsies, researchers analyzed brain tissue markers related to Alzheimer’s and found an association with these indicators. In 2022, Ryan Powell, the lead author of that study, published further findings that neighborhood disadvantage was connected with having more neurofibrillary tangles and amyloid plaques, the main pathological features of Alzheimer's disease.
As of yet, little is known about the biological processes behind this, says Powell, director of data science at the Center for Health Disparities Research at the University of Wisconsin School of Medicine and Public Health. “We know the association but not the direct causal pathway.”
The corroborative findings keep coming. In a Nature study published a few months after Powell’s study, every social determinant investigated affected Alzheimer’s risk except for marital status. The links were highest for income, education, and occupational status.
Clinical trials on new Alzheimer’s medications get all the headlines but preventing dementia through policy and public health interventions should not be underestimated.
The potential for prevention is significant. One in three older adults dies with Alzheimer's or another dementia—more than breast and prostate cancers combined. Further, a 2020 report from the Lancet Commission determined that about 40 percent of dementia cases could theoretically be prevented or delayed by managing the risk factors that people can modify.
Take inactivity. Older adults who took 9,800 steps daily were half as likely to develop dementia over the next 7 years, in a 2022 JAMA study. Hearing loss, another risk factor that can be managed, accounts for about 9 percent of dementia cases.
Clinical trials on new Alzheimer’s medications get all the headlines but preventing dementia through policy and public health interventions should not be underestimated. Simply slowing the course of Alzheimer’s or delaying its onset by five years would cut the incidence in half, according to the Global Council on Brain Health.
Minorities Hit the Hardest
The World Health Organization defines SDOH as “conditions in which people are born, work, live, and age, and the wider set of forces and systems shaping the conditions of daily life.”
Anyone who exists on processed food, smokes cigarettes, or skimps on sleep has heightened risks for dementia. But minority groups get hit harder. Older Black Americans are twice as likely to have Alzheimer’s or another form of dementia as white Americans; older Hispanics are about one and a half times more likely.
This is due in part to higher rates of diabetes, obesity, and high blood pressure within these communities. These diseases are linked to Alzheimer’s, and SDOH factors multiply the risks. Blacks and Hispanics earn less income on average than white people. This means they are more likely to live in neighborhoods with limited access to healthy food, medical care, and good schools, and suffer greater exposure to noise (which impairs hearing) and air pollution—additional risk factors for dementia.
Related Reading: The Toxic Effects of Noise and What We're Not Doing About it
Plus, when Black people are diagnosed with dementia, their cognitive impairment and neuropsychiatric symptom are more advanced than in white patients. Why? Some African-Americans delay seeing a doctor because of perceived discrimination and a sense they will not be heard, says Carl V. Hill, chief diversity, equity, and inclusion officer at the Alzheimer’s Association.
Misinformation about dementia is another issue in Black communities. The thinking is that Alzheimer’s is genetic or age-related, not realizing that diet and physical activity can improve brain health, Hill says.
African Americans are severely underrepresented in clinical trials for Alzheimer’s, too. So, researchers miss the opportunity to learn more about health disparities. “It’s a bioethical issue,” Hill says. “The people most likely to have Alzheimer’s aren’t included in the trials.”
The Cure: Systemic Change
People think of lifestyle as a choice but there are limitations, says Muniza Anum Majoka, a geriatric psychiatrist and assistant professor of psychiatry at Yale University, who published an overview of SDOH factors that impact dementia. “For a lot of people, those choices [to improve brain health] are not available,” she says. If you don’t live in a safe neighborhood, for example, walking for exercise is not an option.
Hill wants to see the focus of prevention shift from individual behavior change to ensuring everyone has access to the same resources. Advice about healthy eating only goes so far if someone lives in a food desert. Systemic change also means increasing the number of minority physicians and recruiting minorities in clinical drug trials so studies will be relevant to these communities, Hill says.
Based on SDOH impact research, raising education levels has the most potential to prevent dementia. One theory is that highly educated people have a greater brain reserve that enables them to tolerate pathological changes in the brain, thus delaying dementia, says Majoka. Being curious, learning new things and problem-solving also contribute to brain health, she adds. Plus, having more education may be associated with higher socioeconomic status, more access to accurate information and healthier lifestyle choices.
New Strategies
The chasm between what researchers know about brain health and how the knowledge is being applied is huge. “There’s an explosion of interest in this area. We’re just in the first steps,” says Powell. One day, he predicts that physicians will manage Alzheimer’s through precision medicine customized to the patient’s specific risk factors and needs.
Raina Croff, assistant professor of neurology at Oregon Health & Science University School of Medicine, created the SHARP (Sharing History through Active Reminiscence and Photo-imagery) walking program to forestall memory loss in African Americans with mild cognitive impairment or early dementia.
Participants and their caregivers walk in historically black neighborhoods three times a week over six months. A smart tablet provides information about “Memory Markers” they pass, such as the route of a civil rights march. People celebrate their community and culture while “brain health is running in the background,” Croff says.

Photos and memory prompts engage participants in the SHARP program.
OHSU/Kristyna Wentz-Graff
The project began in 2015 as a pilot study in Croff’s hometown of Portland, Ore., expanded to Seattle, and will soon start in Oakland, Calif. “Walking is good for slowing [brain] decline,” she says. A post-study assessment of 40 participants in 2017 showed that half had higher cognitive scores after the program; 78 percent had lower blood pressure; and 44 percent lost weight. Those with mild cognitive impairment showed the most gains. The walkers also reported improved mood and energy along with increased involvement in other activities.
It’s never too late to reap the benefits of working your brain and being socially engaged, Majoka says.
In Milwaukee, the Wisconsin Alzheimer’s Institute launched the The Amazing Grace Chorus® to stave off cognitive decline in seniors. People in early stages of Alzheimer’s practice and perform six concerts each year. The activity provides opportunities for social engagement, mental stimulation, and a support network. Among the benefits, 55 percent reported better communication at home and nearly half of participants said they got involved with more activities after participating in the chorus.
Private companies are offering intervention services to healthcare providers and insurers to manage SDOH, too. One such service, MyHello, makes calls to at-risk people to assess their needs—be it food, transportation or simply a friendly voice. Having a social support network is critical for seniors, says Majoka, noting there was a steep decline in cognitive function among isolated elders during Covid lockdowns.
About 1 in 9 Americans age 65 or older live with Alzheimer’s today. With a surge in people with the disease predicted, public health professionals have to think more broadly about resource targets and effective intervention points, Powell says.
Beyond breakthrough pills, that is. Like Dorothy in Kansas discovering happiness was always in her own backyard, we are beginning to learn that preventing Alzheimer’s is in our reach if only we recognized it.
A model of a human kidney.
Science's dream of creating perfect custom organs on demand as soon as a patient needs one is still a long way off. But tiny versions are already serving as useful research tools and stepping stones toward full-fledged replacements.
Although organoids cannot yet replace kidneys, they are invaluable tools for research.
The Lowdown
Australian researchers have grown hundreds of mini human kidneys in the past few years. Known as organoids, they function much like their full-grown counterparts, minus a few features due to a lack of blood supply.
Cultivated in a petri dish, these kidneys are still a shadow of their human counterparts. They grow no larger than one-sixth of an inch in diameter; fully developed organs are up to five inches in length. They contain no more than a few dozen nephrons, the kidney's individual blood-filtering unit, whereas a fully-grown kidney has about 1 million nephrons. And the dish variety live for just a few weeks.
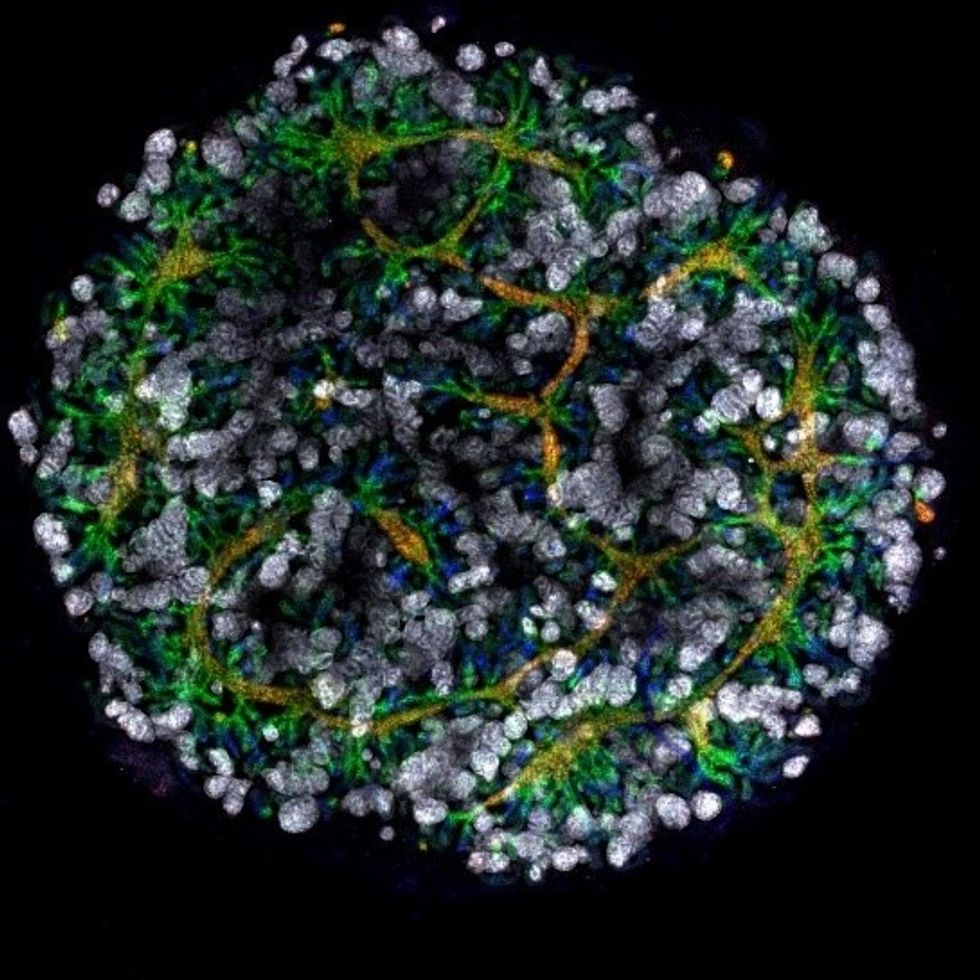
An organoid kidney created by the Murdoch Children's Institute in Melbourne, Australia.
Photo Credit: Shahnaz Khan.
But Melissa Little, head of the kidney research laboratory at the Murdoch Children's Institute in Melbourne, says these organoids are invaluable tools for research. Although renal failure is rare in children, more than half of those who suffer from such a disorder inherited it.
The mini kidneys enable scientists to better understand the progression of such disorders because they can be grown with a patient's specific genetic condition.
Mature stem cells can be extracted from a patient's blood sample and then reprogrammed to become like embryonic cells, able to turn into any type of cell in the body. It's akin to walking back the clock so that the cells regain unlimited potential for development. (The Japanese scientist who pioneered this technique was awarded the Nobel Prize in 2012.) These "induced pluripotent stem cells" can then be chemically coaxed to grow into mini kidneys that have the patient's genetic disorder.
"The (genetic) defects are quite clear in the organoids, and they can be monitored in the dish," Little says. To date, her research team has created organoids from 20 different stem cell lines.
Medication regimens can also be tested on the organoids, allowing specific tailoring for each patient. For now, such testing remains restricted to mice, but Little says it eventually will be done on human organoids so that the results can more accurately reflect how a given patient will respond to particular drugs.
Next Steps
Although these organoids cannot yet replace kidneys, Little says they may plug a huge gap in renal care by assisting in developing new treatments for chronic conditions. Currently, most patients with a serious kidney disorder see their options narrow to dialysis or organ transplantation. The former not only requires multiple sessions a week, but takes a huge toll on patient health.
Ten percent of older patients on dialysis die every year in the U.S. Aside from the physical trauma of organ transplantation, finding a suitable donor outside of a family member can be difficult.
"This is just another great example of the potential of pluripotent stem cells."
Meanwhile, the ongoing creation of organoids is supplying Little and her colleagues with enough information to create larger and more functional organs in the future. According to Little, researchers in the Netherlands, for example, have found that implanting organoids in mice leads to the creation of vascular growth, a potential pathway toward creating bigger and better kidneys.
And while Little acknowledges that creating a fully-formed custom organ is the ultimate goal, the mini organs are an important bridge step.
"This is just another great example of the potential of pluripotent stem cells, and I am just passionate to see it do some good."
Phil Gutis, an Alzheimer's patient who participated in a failed clinical trial, poses with his dog Abe.
Phil Gutis never had a stellar memory, but when he reached his early 50s, it became a problem he could no longer ignore. He had trouble calculating how much to tip after a meal, finding things he had just put on his desk, and understanding simple driving directions.
From 1998-2017, industry sources reported 146 failed attempts at developing Alzheimer's drugs.
So three years ago, at age 54, he answered an ad for a drug trial seeking people experiencing memory issues. He scored so low in the memory testing he was told something was wrong. M.R.I.s and PET scans confirmed that he had early-onset Alzheimer's disease.
Gutis, who is a former New York Times reporter and American Civil Liberties Union spokesman, felt fortunate to get into an advanced clinical trial of a new treatment for Alzheimer's disease. The drug, called aducanumab, had shown promising results in earlier studies.
Four years of data had found that the drug effectively reduced the burden of protein fragments called beta-amyloids, which destroy connections between nerve cells. Amyloid plaques are found in the brains of patients with Alzheimer's disease and are associated with impairments in thinking and memory.
Gutis eagerly participated in the clinical trial and received 35 monthly infusions. "For the first 20 infusions, I did not know whether I was receiving the drug or the placebo," he says. "During the last 15 months, I received aducanumab. But it really didn't matter if I was receiving the drug or the placebo because on March 21, the trial was stopped because [the drug company] Biogen found that the treatments were ineffective."
The news was devastating to the trial participants, but also to the Alzheimer's research community. Earlier this year, another pharmaceutical company, Roche, announced it was discontinuing two of its Alzheimer's clinical trials. From 1998-2017, industry sources reported 146 failed attempts at developing Alzheimer's drugs. There are five prescription drugs approved to treat its symptoms, but a cure remains elusive. The latest failures have left researchers scratching their heads about how to approach attacking the disease.
The failure of aducanumab was also another setback for the estimated 5.8 million people who have Alzheimer's in the United States. Of these, around 5.6 million are older than 65 and 200,000 suffer from the younger-onset form, including Gutis.
Gutis is understandably distraught about the cancellation of the trial. "I really had hopes it would work. So did all the patients."
While drug companies have failed so far, another group is stepping up to expedite the development of a cure: venture philanthropists.
For now, he is exercising every day to keep his blood flowing, which is supposed to delay the progression of the disease, and trying to eat a low-fat diet. "But I know that none of it will make a difference. Alzheimer's is a progressive disease. There are no treatments to delay it, let alone cure it."
But while drug companies have failed so far, another group is stepping up to expedite the development of a cure: venture philanthropists. These are successful titans of industry and dedicated foundations who are donating large sums of money to fill a much-needed void – funding research to look for new biomarkers.
Biomarkers are neurochemical indicators that can be used to detect the presence of a disease and objectively measure its progression. There are currently no validated biomarkers for Alzheimer's, but researchers are actively studying promising candidates. The hope is that they will find a reliable way to identify the disease even before the symptoms of mental decline show up, so that treatments can be directed at a very early stage.
Howard Fillit, Founding Executive Director and Chief Science Officer of the Alzheimer's Drug Discovery Foundation, says, "We need novel biomarkers to diagnose Alzheimer's disease and related dementias. But pharmaceutical companies don't put money into biomarkers research."
One of the venture philanthropists who has recently stepped up to the task is Bill Gates. In January 2018, he announced his father had Alzheimer's disease in an interview on the Today Show with Maria Shriver, whose father Sargent Shriver, died of Alzheimer's disease in 2011. Gates told Ms. Shriver that he had invested $100 million into Alzheimer's research, with $50 million of his donation going to Dementia Discovery Fund, which looks for new cures and treatments.
That August, Gates joined other investors in a new fund called Diagnostics Accelerator. The project aims to supports researchers looking to speed up new ideas for earlier and better diagnosis of the disease.
Gates and other donors committed more than $35 million to help launch it, and this April, Jeff and Mackenzie Bezos joined the coalition, bringing the current program funding to nearly $50 million.
"It makes sense that a challenge this significant would draw the attention of some of the world's leading thinkers."
None of these funders stand to make a profit on their donation, unlike traditional research investments by drug companies. The standard alternatives to such funding have upsides -- and downsides.
As Bill Gates wrote on his blog, "Investments from governments or charitable organizations are fantastic at generating new ideas and cutting-edge research -- but they're not always great at creating usable products, since no one stands to make a profit at the end of the day.
"Venture capital, on the other end of the spectrum, is more likely to develop a test that will reach patients, but its financial model favors projects that will earn big returns for investors. Venture philanthropy splits the difference. It incentivizes a bold, risk-taking approach to research with an end goal of a real product for real patients. If any of the projects backed by Diagnostics Accelerator succeed, our share of the financial windfall goes right back into the fund."
Gutis said he is thankful for any attention given to finding a cure for Alzheimer's.
"Most doctors and scientists will tell you that we're still in the dark ages when it comes to fully understanding how the brain works, let alone figuring out the cause or treatment for Alzheimer's.
"It makes sense that a challenge this significant would draw the attention of some of the world's leading thinkers. I only hope they can be more successful with their entrepreneurial approach to finding a cure than the drug companies have been with their more traditional paths."