New tools could catch disease outbreaks earlier - or predict them

A team at the Catalan Institution for Research and Advanced Studies is utilizing drones and weather stations to collect data on how mosquito breeding patterns are changing in response to climate shifts.
Every year, the villages which lie in the so-called ‘Nipah belt’— which stretches along the western border between Bangladesh and India, brace themselves for the latest outbreak. For since 1998, when Nipah virus—a form of hemorrhagic fever most common in Bangladesh—first spilled over into humans, it has been a grim annual visitor to the people of this region.
With a 70 percent fatality rate, no vaccine, and no known treatments, Nipah virus has been dubbed in the Western world as ‘the worst disease no one has ever heard of.’ Currently, outbreaks tend to be relatively contained because it is not very transmissible. The virus circulates throughout Asia in fruit eating bats, and only tends to be passed on to people who consume contaminated date palm sap, a sweet drink which is harvested across Bangladesh.
But as SARS-CoV-2 has shown the world, this can quickly change.
“Nipah virus is among what virologists call ‘the Big 10,’ along with things like Lassa fever and Crimean Congo hemorrhagic fever,” says Noam Ross, a disease ecologist at New York-based non-profit EcoHealth Alliance. “These are pretty dangerous viruses from a lethality perspective, which don’t currently have the capacity to spread into broader human populations. But that can evolve, and you could very well see a variant emerge that has human-human transmission capability.”
That’s not an overstatement. Surveys suggest that mammals harbour about 40,000 viruses, with roughly a quarter capable of infecting humans. The vast majority never get a chance to do so because we don’t encounter them, but climate change can alter that. Recent studies have found that as animals relocate to new habitats due to shifting environmental conditions, the coming decades will bring around 300,000 first encounters between species which normally don’t interact, especially in tropical Africa and southeast Asia. All these interactions will make it far more likely for hitherto unknown viruses to cross paths with humans.
That’s why for the last 16 years, EcoHealth Alliance has been conducting ongoing viral surveillance projects across Bangladesh. The goal is to understand why Nipah is so much more prevalent in the western part of the country, compared to the east, and keep a watchful eye out for new Nipah strains as well as other dangerous pathogens like Ebola.
"There are a lot of different infectious agents that are sensitive to climate change that don't have these sorts of software tools being developed for them," says Cat Lippi, medical geography researcher at the University of Florida.
Until very recently this kind of work has been hampered by the limitations of viral surveillance technology. The PREDICT project, a $200 million initiative funded by the United States Agency for International Development, which conducted surveillance across the Amazon Basin, Congo Basin and extensive parts of South and Southeast Asia, relied upon so-called nucleic acid assays which enabled scientists to search for the genetic material of viruses in animal samples.
However, the project came under criticism for being highly inefficient. “That approach requires a big sampling effort, because of the rarity of individual infections,” says Ross. “Any particular animal may be infected for a couple of weeks, maybe once or twice in its lifetime. So if you sample thousands and thousands of animals, you'll eventually get one that has an Ebola virus infection right now.”
Ross explains that there is now far more interest in serological sampling—the scientific term for the process of drawing blood for antibody testing. By searching for the presence of antibodies in the blood of humans and animals, scientists have a greater chance of detecting viruses which started circulating recently.
Despite the controversy surrounding EcoHealth Alliance’s involvement in so-called gain of function research—experiments that study whether viruses might mutate into deadlier strains—the organization’s separate efforts to stay one step ahead of pathogen evolution are key to stopping the next pandemic.
“Having really cheap and fast surveillance is really important,” says Ross. “Particularly in a place where there's persistent, low level, moderate infections that potentially have the ability to develop into more epidemic or pandemic situations. It means there’s a pathway that something more dangerous can come through."
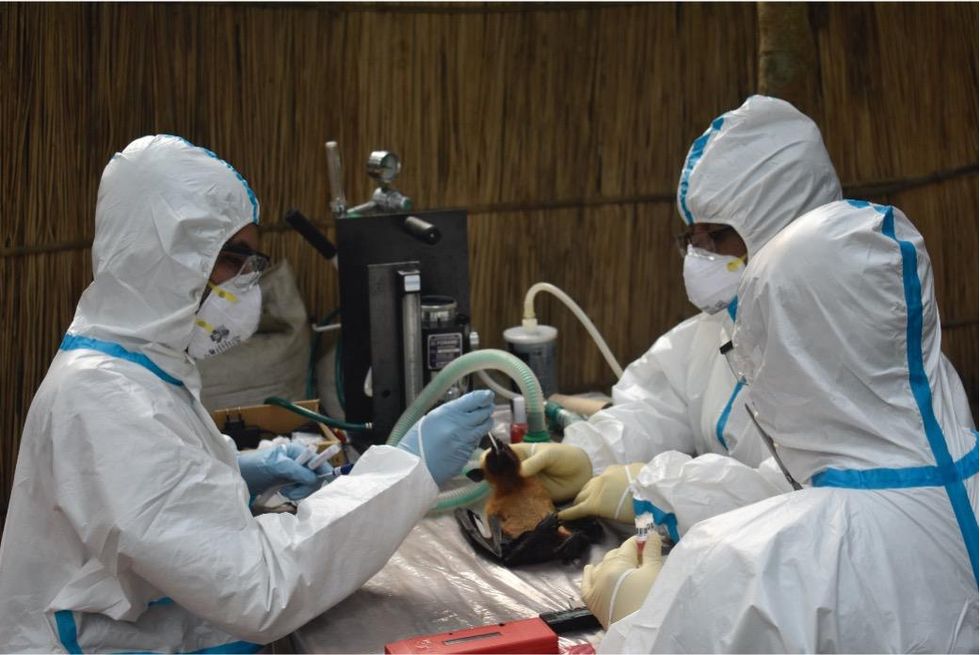
Scientists are searching for the presence of antibodies in the blood of humans and animals in hopes to detect viruses that recently started circulating.
EcoHealth Alliance
In Bangladesh, EcoHealth Alliance is attempting to do this using a newer serological technology known as a multiplex Luminex assay, which tests samples against a panel of known antibodies against many different viruses. It collects what Ross describes as a ‘footprint of information,’ which allows scientists to tell whether the sample contains the presence of a known pathogen or something completely different and needs to be investigated further.
By using this technology to sample human and animal populations across the country, they hope to gain an idea of whether there are any novel Nipah virus variants or strains from the same family, as well as other deadly viral families like Ebola.
This is just one of several novel tools being used for viral discovery in surveillance projects around the globe. Multiple research groups are taking PREDICT’s approach of looking for novel viruses in animals in various hotspots. They collect environmental DNA—mucus, faeces or shed skin left behind in soil, sediment or water—which can then be genetically sequenced.
Five years ago, this would have been a painstaking work requiring bringing collected samples back to labs. Today, thanks to the vast amounts of money spent on new technologies during COVID-19, researchers now have portable sequencing tools they can take out into the field.
Christopher Jerde, a researcher at the UC Santa Barbara Marine Science Institute, points to the Oxford Nanopore MinION sequencer as one example. “I tried one of the early versions of it four years ago, and it was miserable,” he says. “But they’ve really improved, and what we’re going to be able to do in the next five to ten years will be amazing. Instead of having to carefully transport samples back to the lab, we're going to have cigar box-shaped sequencers that we take into the field, plug into a laptop, and do the whole sequencing of an organism.”
In the past, viral surveillance has had to be very targeted and focused on known families of viruses, potentially missing new, previously unknown zoonotic pathogens. Jerde says that the rise of portable sequencers will lead to what he describes as “true surveillance.”
“Before, this was just too complex,” he says. “It had to be very focused, for example, looking for SARS-type viruses. Now we’re able to say, ‘Tell us all the viruses that are here?’ And this will give us true surveillance – we’ll be able to see the diversity of all the pathogens which are in these spots and have an understanding of which ones are coming into the population and causing damage.”
But being able to discover more viruses also comes with certain challenges. Some scientists fear that the speed of viral discovery will soon outpace the human capacity to analyze them all and assess the threat that they pose to us.
“I think we're already there,” says Jason Ladner, assistant professor at Northern Arizona University’s Pathogen and Microbiome Institute. “If you look at all the papers on the expanding RNA virus sphere, there are all of these deposited partial or complete viral sequences in groups that we just don't know anything really about yet.” Bats, for example, carry a myriad of viruses, whose ability to infect human cells we understand very poorly.
Cultivating these viruses under laboratory conditions and testing them on organoids— miniature, simplified versions of organs created from stem cells—can help with these assessments, but it is a slow and painstaking work. One hope is that in the future, machine learning could help automate this process. The new SpillOver Viral Risk Ranking platform aims to assess the risk level of a given virus based on 31 different metrics, while other computer models have tried to do the same based on the similarity of a virus’s genomic sequence to known zoonotic threats.
However, Ladner says that these types of comparisons are still overly simplistic. For one thing, scientists are still only aware of a few hundred zoonotic viruses, which is a very limited data sample for accurately assessing a novel pathogen. Instead, he says that there is a need for virologists to develop models which can determine viral compatibility with human cells, based on genomic data.
“One thing which is really useful, but can be challenging to do, is understand the cell surface receptors that a given virus might use,” he says. “Understanding whether a virus is likely to be able to use proteins on the surface of human cells to gain entry can be very informative.”
As the Earth’s climate heats up, scientists also need to better model the so-called vector borne diseases such as dengue, Zika, chikungunya and yellow fever. Transmitted by the Aedes mosquito residing in humid climates, these blights currently disproportionally affect people in low-income nations. But predictions suggest that as the planet warms and the pests find new homes, an estimated one billion people who currently don’t encounter them might be threatened by their bites by 2080. “When it comes to mosquito-borne diseases we have to worry about shifts in suitable habitat,” says Cat Lippi, a medical geography researcher at the University of Florida. “As climate patterns change on these big scales, we expect to see shifts in where people will be at risk for contracting these diseases.”
Public health practitioners and government decision-makers need tools to make climate-informed decisions about the evolving threat of different infectious diseases. Some projects are already underway. An ongoing collaboration between the Catalan Institution for Research and Advanced Studies and researchers in Brazil and Peru is utilizing drones and weather stations to collect data on how mosquitoes change their breeding patterns in response to climate shifts. This information will then be fed into computer algorithms to predict the impact of mosquito-borne illnesses on different regions.

The team at the Catalan Institution for Research and Advanced Studies is using drones and weather stations to collect data on how mosquito breeding patterns change due to climate shifts.
Gabriel Carrasco
Lippi says that similar models are urgently needed to predict how changing climate patterns affect respiratory, foodborne, waterborne and soilborne illnesses. The UK-based Wellcome Trust has allocated significant assets to fund such projects, which should allow scientists to monitor the impact of climate on a much broader range of infections. “There are a lot of different infectious agents that are sensitive to climate change that don't have these sorts of software tools being developed for them,” she says.
COVID-19’s havoc boosted funding for infectious disease research, but as its threats begin to fade from policymakers’ focus, the money may dry up. Meanwhile, scientists warn that another major infectious disease outbreak is inevitable, potentially within the next decade, so combing the planet for pathogens is vital. “Surveillance is ultimately a really boring thing that a lot of people don't want to put money into, until we have a wide scale pandemic,” Jerde says, but that vigilance is key to thwarting the next deadly horror. “It takes a lot of patience and perseverance to keep looking.”
This article originally appeared in One Health/One Planet, a single-issue magazine that explores how climate change and other environmental shifts are increasing vulnerabilities to infectious diseases by land and by sea. The magazine probes how scientists are making progress with leaders in other fields toward solutions that embrace diverse perspectives and the interconnectedness of all lifeforms and the planet.
The Voice Behind Some of Your Favorite Cartoon Characters Helped Create the Artificial Heart
This Jarvik-7 artificial heart was used in the first bridge operation in 1985 meant to replace a failing heart while the patient waited for a donor organ.
In June, a team of surgeons at Duke University Hospital implanted the latest model of an artificial heart in a 39-year-old man with severe heart failure, a condition in which the heart doesn't pump properly. The man's mechanical heart, made by French company Carmat, is a new generation artificial heart and the first of its kind to be transplanted in the United States. It connects to a portable external power supply and is designed to keep the patient alive until a replacement organ becomes available.
Many patients die while waiting for a heart transplant, but artificial hearts can bridge the gap. Though not a permanent solution for heart failure, artificial hearts have saved countless lives since their first implantation in 1982.
What might surprise you is that the origin of the artificial heart dates back decades before, when an inventive television actor teamed up with a famous doctor to design and patent the first such device.
A man of many talents
Paul Winchell was an entertainer in the 1950s and 60s, rising to fame as a ventriloquist and guest-starring as an actor on programs like "The Ed Sullivan Show" and "Perry Mason." When children's animation boomed in the 1960s, Winchell made a name for himself as a voice actor on shows like "The Smurfs," "Winnie the Pooh," and "The Jetsons." He eventually became famous for originating the voices of Tigger from "Winnie the Pooh" and Gargamel from "The Smurfs," among many others.
But Winchell wasn't just an entertainer: He also had a quiet passion for science and medicine. Between television gigs, Winchell busied himself working as a medical hypnotist and acupuncturist, treating the same Hollywood stars he performed alongside. When he wasn't doing that, Winchell threw himself into engineering and design, building not only the ventriloquism dummies he used on his television appearances but a host of products he'd dreamed up himself. Winchell spent hours tinkering with his own inventions, such as a set of battery-powered gloves and something called a "flameless lighter." Over the course of his life, Winchell designed and patented more than 30 of these products – mostly novelties, but also serious medical devices, such as a portable blood plasma defroster.

| Ventriloquist Paul Winchell with Jerry Mahoney, his dummy, in 1951 |
A meeting of the minds
In the early 1950s, Winchell appeared on a variety show called the "Arthur Murray Dance Party" and faced off in a dance competition with the legendary Ricardo Montalban (Winchell won). At a cast party for the show later that same night, Winchell met Dr. Henry Heimlich – the same doctor who would later become famous for inventing the Heimlich maneuver, who was married to Murray's daughter. The two hit it off immediately, bonding over their shared interest in medicine. Before long, Heimlich invited Winchell to come observe him in the operating room at the hospital where he worked. Winchell jumped at the opportunity, and not long after he became a frequent guest in Heimlich's surgical theatre, fascinated by the mechanics of the human body.
One day while Winchell was observing at the hospital, he witnessed a patient die on the operating table after undergoing open-heart surgery. He was suddenly struck with an idea: If there was some way doctors could keep blood pumping temporarily throughout the body during surgery, patients who underwent risky operations like open-heart surgery might have a better chance of survival. Winchell rushed to Heimlich with the idea – and Heimlich agreed to advise Winchell and look over any design drafts he came up with. So Winchell went to work.
Winchell's heart
As it turned out, building ventriloquism dummies wasn't that different from building an artificial heart, Winchell noted later in his autobiography – the shifting valves and chambers of the mechanical heart were similar to the moving eyes and opening mouths of his puppets. After each design, Winchell would go back to Heimlich and the two would confer, making adjustments along the way to.
By 1956, Winchell had perfected his design: The "heart" consisted of a bag that could be placed inside the human body, connected to a battery-powered motor outside of the body. The motor enabled the bag to pump blood throughout the body, similar to a real human heart. Winchell received a patent for the design in 1963.
At the time, Winchell never quite got the credit he deserved. Years later, researchers at the University of Utah, working on their own artificial heart, came across Winchell's patent and got in touch with Winchell to compare notes. Winchell ended up donating his patent to the team, which included Dr. Richard Jarvik. Jarvik expanded on Winchell's design and created the Jarvik-7 – the world's first artificial heart to be successfully implanted in a human being in 1982.
The Jarvik-7 has since been replaced with newer, more efficient models made up of different synthetic materials, allowing patients to live for longer stretches without the heart clogging or breaking down. With each new generation of hearts, heart failure patients have been able to live relatively normal lives for longer periods of time and with fewer complications than before – and it never would have been possible without the unsung genius of a puppeteer and his love of science.
Elaine Kamil had just returned home after a few days of business meetings in 2013 when she started having chest pains. At first Kamil, then 66, wasn't worried—she had had some chest pain before and recently went to a cardiologist to do a stress test, which was normal.
"I can't be having a heart attack because I just got checked," she thought, attributing the discomfort to stress and high demands of her job. A pediatric nephrologist at Cedars-Sinai Hospital in Los Angeles, she takes care of critically ill children who are on dialysis or are kidney transplant patients. Supporting families through difficult times and answering calls at odd hours is part of her daily routine, and often leaves her exhausted.
She figured the pain would go away. But instead, it intensified that night. Kamil's husband drove her to the Cedars-Sinai hospital, where she was admitted to the coronary care unit. It turned out she wasn't having a heart attack after all. Instead, she was diagnosed with a much less common but nonetheless dangerous heart condition called takotsubo syndrome, or broken heart syndrome.
A heart attack happens when blood flow to the heart is obstructed—such as when an artery is blocked—causing heart muscle tissue to die. In takotsubo syndrome, the blood flow isn't blocked, but the heart doesn't pump it properly. The heart changes its shape and starts to resemble a Japanese fishing device called tako-tsubo, a clay pot with a wider body and narrower mouth, used to catch octopus.
"The heart muscle is stunned and doesn't function properly anywhere from three days to three weeks," explains Noel Bairey Merz, the cardiologist at Cedar Sinai who Kamil went to see after she was discharged.
"The heart muscle is stunned and doesn't function properly anywhere from three days to three weeks."
But even though the heart isn't permanently damaged, mortality rates due to takotsubo syndrome are comparable to those of a heart attack, Merz notes—about 4-5% of patients die from the attack, and 20% within the next five years. "It's as bad as a heart attack," Merz says—only it's much less known, even to doctors. The condition affects only about 1% of people, and there are around 15,000 new cases annually. It's diagnosed using a cardiac ventriculogram, an imaging test that allows doctors to see how the heart pumps blood.
Scientists don't fully understand what causes Takotsubo syndrome, but it usually occurs after extreme emotional or physical stress. Doctors think it's triggered by a so-called catecholamine storm, a phenomenon in which the body releases too much catecholamines—hormones involved in the fight-or-flight response. Evolutionarily, when early humans lived in savannas or forests and had to either fight off predators or flee from them, these hormones gave our ancestors the needed strength and stamina to take either action. Released by nerve endings and by the adrenal glands that sit on top of the kidneys, these hormones still flood our bodies in moments of stress, but an overabundance of them could sometimes be damaging.

Elaine Kamil
A recent study by scientists at Harvard Medical School linked increased risk of takotsubo to higher activity in the amygdala, a brain region responsible for emotions that's involved in responses to stress. The scientists believe that chronic stress makes people more susceptible to the syndrome. Notably, one small study suggested that the number of Takotsubo cases increased during the COVID-19 pandemic.
There are no specific drugs to treat takotsubo, so doctors rely on supportive therapies, which include medications typically used for high blood pressure and heart failure. In most cases, the heart returns to its normal shape within a few weeks. "It's a spontaneous recovery—the catecholamine storm is resolved, the injury trigger is removed and the heart heals itself because our bodies have an amazing healing capacity," Merz says. It also helps that tissues remain intact. 'The heart cells don't die, they just aren't functioning properly for some time."
That's the good news. The bad news is that takotsubo is likely to strike again—in 5-20% of patients the condition comes back, sometimes more severe than before.
That's exactly what happened to Kamil. After getting her diagnosis in 2013, she realized that she actually had a previous takotsubo episode. In 2010, she experienced similar symptoms after her son died. "The night after he died, I was having severe chest pain at night, but I was too overwhelmed with grief to do anything about it," she recalls. After a while, the pain subsided and didn't return until three years later.
For weeks after her second attack, she felt exhausted, listless and anxious. "You lose confidence in your body," she says. "You have these little twinges on your chest, or if you start having arrhythmia, and you wonder if this is another episode coming up. It's really unnerving because you don't know how to read these cues." And that's very typical, Merz says. Even when the heart muscle appears to recover, patients don't return to normal right away. They have shortens of breath, they can't exercise, and they stay anxious and worried for a while.
Women over the age of 50 are diagnosed with takotsubo more often than other demographics. However, it happens in men too, although it typically strikes after physical stress, such as a triathlon or an exhausting day of cycling. Young people can also get takotsubo. Older patients are hospitalized more often, but younger people tend to have more severe complications. It could be because an older person may go for a jog while younger one may run a marathon, which would take a stronger toll on the body of a person who's predisposed to the condition.
Notably, the emotional stressors don't always have to be negative—the heart muscle can get out of shape from good emotions, too. "There have been case reports of takotsubo at weddings," Merz says. Moreover, one out of three or four takotsubo patients experience no apparent stress, she adds. "So it could be that it's not so much the catecholamine storm itself, but the body's reaction to it—the physiological reaction deeply embedded into out physiology," she explains.
Merz and her team are working to understand what makes people predisposed to takotsubo. They think a person's genetics play a role, but they haven't yet pinpointed genes that seem to be responsible. Genes code for proteins, which affect how the body metabolizes various compounds, which, in turn, affect the body's response to stress. Pinning down the protein involved in takotsubo susceptibility would allow doctors to develop screening tests and identify those prone to severe repeating attacks. It will also help develop medications that can either prevent it or treat it better than just waiting for the body to heal itself.
Researchers at the Imperial College London recently found that elevated levels of certain types of microRNAs—molecules involved in protein production—increase the chances of developing takotsubo.
In one study, researchers tried treating takotsubo in mice with a drug called suberanilohydroxamic acid, or SAHA, typically used for cancer treatment. The drug improved cardiac health and reversed the broken heart in rodents. It remains to be seen if the drug would have a similar effect on humans. But identifying a drug that shows promise is progress, Merz says. "I'm glad that there's research in this area."
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.

