New tools could catch disease outbreaks earlier - or predict them

A team at the Catalan Institution for Research and Advanced Studies is utilizing drones and weather stations to collect data on how mosquito breeding patterns are changing in response to climate shifts.
Every year, the villages which lie in the so-called ‘Nipah belt’— which stretches along the western border between Bangladesh and India, brace themselves for the latest outbreak. For since 1998, when Nipah virus—a form of hemorrhagic fever most common in Bangladesh—first spilled over into humans, it has been a grim annual visitor to the people of this region.
With a 70 percent fatality rate, no vaccine, and no known treatments, Nipah virus has been dubbed in the Western world as ‘the worst disease no one has ever heard of.’ Currently, outbreaks tend to be relatively contained because it is not very transmissible. The virus circulates throughout Asia in fruit eating bats, and only tends to be passed on to people who consume contaminated date palm sap, a sweet drink which is harvested across Bangladesh.
But as SARS-CoV-2 has shown the world, this can quickly change.
“Nipah virus is among what virologists call ‘the Big 10,’ along with things like Lassa fever and Crimean Congo hemorrhagic fever,” says Noam Ross, a disease ecologist at New York-based non-profit EcoHealth Alliance. “These are pretty dangerous viruses from a lethality perspective, which don’t currently have the capacity to spread into broader human populations. But that can evolve, and you could very well see a variant emerge that has human-human transmission capability.”
That’s not an overstatement. Surveys suggest that mammals harbour about 40,000 viruses, with roughly a quarter capable of infecting humans. The vast majority never get a chance to do so because we don’t encounter them, but climate change can alter that. Recent studies have found that as animals relocate to new habitats due to shifting environmental conditions, the coming decades will bring around 300,000 first encounters between species which normally don’t interact, especially in tropical Africa and southeast Asia. All these interactions will make it far more likely for hitherto unknown viruses to cross paths with humans.
That’s why for the last 16 years, EcoHealth Alliance has been conducting ongoing viral surveillance projects across Bangladesh. The goal is to understand why Nipah is so much more prevalent in the western part of the country, compared to the east, and keep a watchful eye out for new Nipah strains as well as other dangerous pathogens like Ebola.
"There are a lot of different infectious agents that are sensitive to climate change that don't have these sorts of software tools being developed for them," says Cat Lippi, medical geography researcher at the University of Florida.
Until very recently this kind of work has been hampered by the limitations of viral surveillance technology. The PREDICT project, a $200 million initiative funded by the United States Agency for International Development, which conducted surveillance across the Amazon Basin, Congo Basin and extensive parts of South and Southeast Asia, relied upon so-called nucleic acid assays which enabled scientists to search for the genetic material of viruses in animal samples.
However, the project came under criticism for being highly inefficient. “That approach requires a big sampling effort, because of the rarity of individual infections,” says Ross. “Any particular animal may be infected for a couple of weeks, maybe once or twice in its lifetime. So if you sample thousands and thousands of animals, you'll eventually get one that has an Ebola virus infection right now.”
Ross explains that there is now far more interest in serological sampling—the scientific term for the process of drawing blood for antibody testing. By searching for the presence of antibodies in the blood of humans and animals, scientists have a greater chance of detecting viruses which started circulating recently.
Despite the controversy surrounding EcoHealth Alliance’s involvement in so-called gain of function research—experiments that study whether viruses might mutate into deadlier strains—the organization’s separate efforts to stay one step ahead of pathogen evolution are key to stopping the next pandemic.
“Having really cheap and fast surveillance is really important,” says Ross. “Particularly in a place where there's persistent, low level, moderate infections that potentially have the ability to develop into more epidemic or pandemic situations. It means there’s a pathway that something more dangerous can come through."
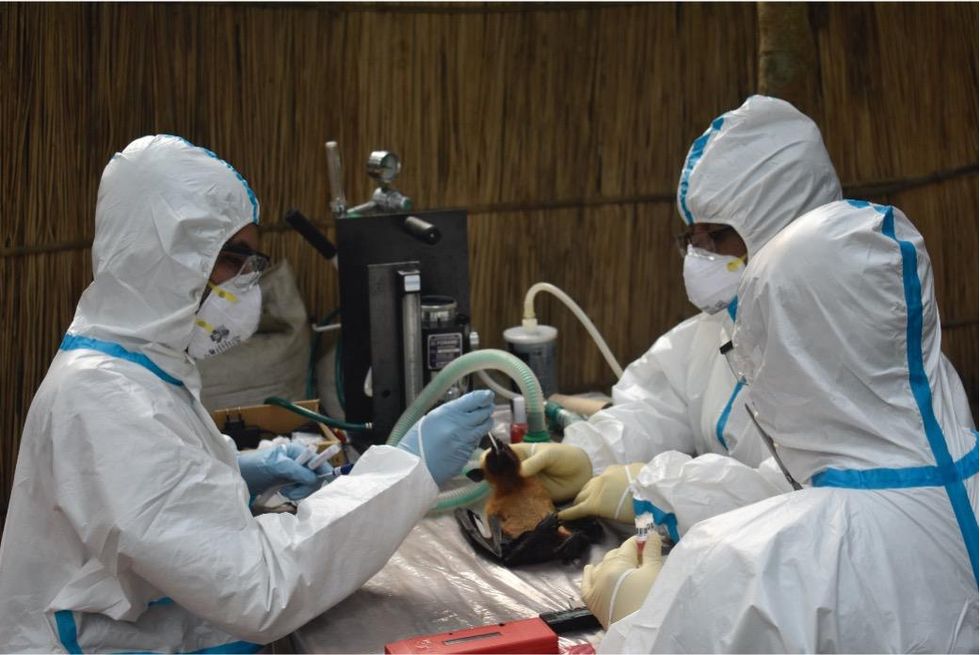
Scientists are searching for the presence of antibodies in the blood of humans and animals in hopes to detect viruses that recently started circulating.
EcoHealth Alliance
In Bangladesh, EcoHealth Alliance is attempting to do this using a newer serological technology known as a multiplex Luminex assay, which tests samples against a panel of known antibodies against many different viruses. It collects what Ross describes as a ‘footprint of information,’ which allows scientists to tell whether the sample contains the presence of a known pathogen or something completely different and needs to be investigated further.
By using this technology to sample human and animal populations across the country, they hope to gain an idea of whether there are any novel Nipah virus variants or strains from the same family, as well as other deadly viral families like Ebola.
This is just one of several novel tools being used for viral discovery in surveillance projects around the globe. Multiple research groups are taking PREDICT’s approach of looking for novel viruses in animals in various hotspots. They collect environmental DNA—mucus, faeces or shed skin left behind in soil, sediment or water—which can then be genetically sequenced.
Five years ago, this would have been a painstaking work requiring bringing collected samples back to labs. Today, thanks to the vast amounts of money spent on new technologies during COVID-19, researchers now have portable sequencing tools they can take out into the field.
Christopher Jerde, a researcher at the UC Santa Barbara Marine Science Institute, points to the Oxford Nanopore MinION sequencer as one example. “I tried one of the early versions of it four years ago, and it was miserable,” he says. “But they’ve really improved, and what we’re going to be able to do in the next five to ten years will be amazing. Instead of having to carefully transport samples back to the lab, we're going to have cigar box-shaped sequencers that we take into the field, plug into a laptop, and do the whole sequencing of an organism.”
In the past, viral surveillance has had to be very targeted and focused on known families of viruses, potentially missing new, previously unknown zoonotic pathogens. Jerde says that the rise of portable sequencers will lead to what he describes as “true surveillance.”
“Before, this was just too complex,” he says. “It had to be very focused, for example, looking for SARS-type viruses. Now we’re able to say, ‘Tell us all the viruses that are here?’ And this will give us true surveillance – we’ll be able to see the diversity of all the pathogens which are in these spots and have an understanding of which ones are coming into the population and causing damage.”
But being able to discover more viruses also comes with certain challenges. Some scientists fear that the speed of viral discovery will soon outpace the human capacity to analyze them all and assess the threat that they pose to us.
“I think we're already there,” says Jason Ladner, assistant professor at Northern Arizona University’s Pathogen and Microbiome Institute. “If you look at all the papers on the expanding RNA virus sphere, there are all of these deposited partial or complete viral sequences in groups that we just don't know anything really about yet.” Bats, for example, carry a myriad of viruses, whose ability to infect human cells we understand very poorly.
Cultivating these viruses under laboratory conditions and testing them on organoids— miniature, simplified versions of organs created from stem cells—can help with these assessments, but it is a slow and painstaking work. One hope is that in the future, machine learning could help automate this process. The new SpillOver Viral Risk Ranking platform aims to assess the risk level of a given virus based on 31 different metrics, while other computer models have tried to do the same based on the similarity of a virus’s genomic sequence to known zoonotic threats.
However, Ladner says that these types of comparisons are still overly simplistic. For one thing, scientists are still only aware of a few hundred zoonotic viruses, which is a very limited data sample for accurately assessing a novel pathogen. Instead, he says that there is a need for virologists to develop models which can determine viral compatibility with human cells, based on genomic data.
“One thing which is really useful, but can be challenging to do, is understand the cell surface receptors that a given virus might use,” he says. “Understanding whether a virus is likely to be able to use proteins on the surface of human cells to gain entry can be very informative.”
As the Earth’s climate heats up, scientists also need to better model the so-called vector borne diseases such as dengue, Zika, chikungunya and yellow fever. Transmitted by the Aedes mosquito residing in humid climates, these blights currently disproportionally affect people in low-income nations. But predictions suggest that as the planet warms and the pests find new homes, an estimated one billion people who currently don’t encounter them might be threatened by their bites by 2080. “When it comes to mosquito-borne diseases we have to worry about shifts in suitable habitat,” says Cat Lippi, a medical geography researcher at the University of Florida. “As climate patterns change on these big scales, we expect to see shifts in where people will be at risk for contracting these diseases.”
Public health practitioners and government decision-makers need tools to make climate-informed decisions about the evolving threat of different infectious diseases. Some projects are already underway. An ongoing collaboration between the Catalan Institution for Research and Advanced Studies and researchers in Brazil and Peru is utilizing drones and weather stations to collect data on how mosquitoes change their breeding patterns in response to climate shifts. This information will then be fed into computer algorithms to predict the impact of mosquito-borne illnesses on different regions.

The team at the Catalan Institution for Research and Advanced Studies is using drones and weather stations to collect data on how mosquito breeding patterns change due to climate shifts.
Gabriel Carrasco
Lippi says that similar models are urgently needed to predict how changing climate patterns affect respiratory, foodborne, waterborne and soilborne illnesses. The UK-based Wellcome Trust has allocated significant assets to fund such projects, which should allow scientists to monitor the impact of climate on a much broader range of infections. “There are a lot of different infectious agents that are sensitive to climate change that don't have these sorts of software tools being developed for them,” she says.
COVID-19’s havoc boosted funding for infectious disease research, but as its threats begin to fade from policymakers’ focus, the money may dry up. Meanwhile, scientists warn that another major infectious disease outbreak is inevitable, potentially within the next decade, so combing the planet for pathogens is vital. “Surveillance is ultimately a really boring thing that a lot of people don't want to put money into, until we have a wide scale pandemic,” Jerde says, but that vigilance is key to thwarting the next deadly horror. “It takes a lot of patience and perseverance to keep looking.”
This article originally appeared in One Health/One Planet, a single-issue magazine that explores how climate change and other environmental shifts are increasing vulnerabilities to infectious diseases by land and by sea. The magazine probes how scientists are making progress with leaders in other fields toward solutions that embrace diverse perspectives and the interconnectedness of all lifeforms and the planet.
Novel Technologies Could Make Coronavirus Vaccines More Stable for Worldwide Shipping
The vaccine from Pfizer will need to be stored at minus 70 degrees Celsius for worldwide distribution.
Ssendi Bosco has long known to fear the rainy season. As deputy health officer of Mubende District, a region in Central Uganda, she is only too aware of the threat that heavy storms can pose to her area's fragile healthcare facilities.
In early October, persistent rain overwhelmed the power generator that supplies electricity to most of the region, causing a blackout for three weeks. The result was that most of Mubende's vaccine supplies against diseases such as tuberculosis, diphtheria, and polio went to waste. "The vaccines need to be constantly refrigerated, so the generator failing means that most of them are now unusable," she says.
This week, the global fight against the coronavirus pandemic received a major boost when Pfizer and their German partner BioNTech released interim results showing that their vaccine has proved more than 90 percent effective at preventing participants in their clinical trial from getting COVID-19.
But while Pfizer has already signed deals to supply the vaccine to the U.S., U.K., Canada, Japan and the European Union, Mubende's recent plight provides an indication of the challenges that distributors will face when attempting to ship a coronavirus vaccine around the globe, particularly to low-income nations.
Experts have estimated that somewhere between 12 billion and 15 billion doses will be needed to immunize the world's population against COVID-19, a staggering scale, and one that has never been attempted before. "The logistics of distributing COVID-19 vaccines have been described as one of the biggest challenges in the history of mankind," says Göran Conradson, managing director of Swedish vaccine manufacturer Ziccum.
But even these estimates do not take into account the potential for vaccine spoilage. Every year, the World Health Organization estimates that over half of the world's vaccines end up being wasted. This happens because vaccines are fragile products. From the moment they are made, to the moment they are administered, they have to be kept within a tightly controlled temperature range. Throughout the entire supply chain – transportation to an airport, the flight to another country, unloaded, distribution via trucks to healthcare facilities, and storage – they must be refrigerated at all times. This is known as the cold chain, and one tiny slip along the way means the vaccines are ruined.
"It's a chain, and any chain is only as strong as its weakest link," says Asel Sartbaeva, a chemist working on vaccine technologies at the University of Bath in the U.K.
For COVID-19, the challenge is even greater because some of the leading vaccine candidates need to be kept at ultracold temperatures. Pfizer's vaccine, for example, must be kept at -70 degrees Celsius, the kind of freezer capabilities rarely found outsides of specialized laboratories. Transporting such a vaccine across North America and Europe will be difficult enough, but supplying it to some of the world's poorest nations in Asia, Africa and South America -- where only 10 percent of healthcare facilities have reliable electricity -- might appear virtually impossible.
But technology may be able to come to the rescue.
Making Vaccines Less Fragile
Just as the world's pharmaceutical companies have been racing against the clock to develop viable COVID-19 vaccine candidates, scientists around the globe have been hastily developing new technologies to try and make vaccines less fragile. Some approaches involve various chemicals that can be added to the vaccine to make them far more resilient to temperature fluctuations during transit, while others focus on insulated storage units that can maintain the vaccine at a certain temperature even if there is a power outage.
Some of these concepts have already been considered for several years, but before COVID-19 there was less of a commercial incentive to bring them to market. "We never felt that there is a need for an investment in this area," explains Sam Kosari, a pharmacist at the University of Canberra, who researches the vaccine cold chain. "Some technologies were developed then to assist with vaccine transport in Africa during Ebola, but since that outbreak was contained, there hasn't been any serious initiative or reward to develop this technology further."
In her laboratory at the University of Bath, Sartbaeva is using silica - the main constituent of sand – to encase the molecular components within a vaccine. Conventional vaccines typically contain protein targets from the virus, which the immune system learns to recognize. However, when they are exposed to temperature changes, these protein structures degrade, and lose their shape, making the vaccine useless. Sartbaeva compares this to how an egg changes its shape and consistency when it is boiled.
When silica is added to a vaccine, it molds to each protein, forming little protective cages around them, and thus preventing them from being affected by temperature changes. "The whole idea is that if we can create a shell around each protein, we can protect it from physically unravelling which is what causes the deactivation of the vaccine," she says.
Other scientists are exploring similar methods of making vaccines more resilient. Researchers at the Jenner Institute at the University of Oxford recently conducted a clinical trial in which they added carbohydrates to a dengue vaccine, to assess whether it became easier to transport.
Both research groups are now hoping to collaborate with the COVID-19 vaccine candidates being developed by AstraZeneca and Imperial College, assuming they become available in 2021.
"It's good we're all working on this big problem, as different methods could work better for different types of COVID-19 vaccines," says Sartbaeva. "I think it will be needed."
Next-Generation Vaccine Technology
While these different technologies could be utilized to try and protect the first wave of COVID-19 vaccines, efforts are also underway to develop completely new methods of vaccination. Much of this research is still in its earliest stages, but it could yield a second generation of COVID-19 vaccine candidates in 2022 and beyond.
"After the first round of mass vaccination, we could well observe regional outbreaks of the disease appearing from time to time in the coming years," says Kosari. "This is the time where new types of vaccines could be helpful."
One novel method being explored by Ziccum and others is dry powder vaccines. The idea is to spray dry the final vaccine into a powder form, where it is more easily preserved and does not require any special cooling while being transported or stored. People then receive the vaccine by inhaling it, rather than having it injected into their bloodstream.
Conradson explains that the concept of dry powder vaccines works on the same principle as dried food products. Because there is no water involved, the vaccine's components are far less affected by temperature changes. "It is actually the water that leads to the destruction of potency of a vaccine when it gets heated," he says. "We're looking to develop a dry powder vaccine for COVID-19 but this will be a second-generation vaccine. At the moment there are more than 200 first-generation candidates, all of which are using conventional technologies due to the timeframe pressures, which I think was the correct decision."
Dry powder COVID-19 vaccines could also be combined with microneedle patches, to allow people to self-administer the vaccine themselves in their own home. Microneedles are miniature needles – measured in millionths of a meter – which are designed to deliver medicines through the skin with minimal pain. So far, they have been used mainly in cosmetic products, but many scientists are working to use them to deliver drugs or vaccines.
At Georgia Institute of Technology in Atlanta, Mark Prausnitz is leading a couple of projects looking at incorporating COVID-19 vaccines into microneedle patches with the hope of running some early-stage clinical trials over the next couple of years. "The advantage is that they maintain the vaccine in a stable, dry state until it dissolves in the skin," he explains.
Prausnitz and others believe that once the first generation of COVID-19 vaccines become available, biotech and pharmaceutical companies will show more interest in adapting their products so they can be used in a dried form or with a microneedle patch. "There is so much pressure to get the COVID vaccine out that right now, vaccine developers are not interested in incorporating a novel delivery method," he says. "That will have to come later, once the pressure is lessened."
The Struggle of Low-Income Nations
For low-income nations, time will only tell whether technological advancements can enable them to access the first wave of licensed COVID-19 vaccines. But reports already suggest that they are in danger of becoming an afterthought in the race to procure vaccine supplies.
While initiatives such as COVAX are attempting to make sure that vaccine access is equitable, high and middle-income countries have already inked deals to secure 3.8 billion doses, with options for another 5 billion. One particularly sobering study by the Duke Global Health Innovation Center has suggested that such hoarding means many low-income nations may not receive a vaccine until 2024.
For Bosco and the residents of Mubende District in Uganda, all they can do is wait. In the meantime, there is a more pressing problem: fixing their generators. "We hope that we can receive a vaccine," she says. "But the biggest problem will be finding ways to safely store it. Right now we cannot keep any medicines or vaccines in the conditions they need, because we don't have the funds to repair our power generators."
Democratic U.S. presidential nominee and former Vice President Joe Biden puts his face mask back on after answering questions following a speech on the effects on the U.S. economy of the Trump administration's response to the coronavirus disease (COVID-19) pandemic during a campaign event in Wilmington, Delaware, U.S., September 4, 2020.
The response to the COVID-19 pandemic will soon become the responsibility of President-elect Biden. As is clear to anyone who honestly looks, the past 10+ months of this pandemic have been a disastrous litany of mistakes, wrong actions, and misinformation.
The result has been the deaths of 240,000 Americans, economic collapse, disruption of routine healthcare, and inability of Americans to pursue their values without fear of contracting or spreading a deadly infectious disease. With the looming change in administration, many proposals will be suggested for the path forward.
Indeed, the Biden campaign published their own plan. This plan encompasses many of the actions my colleagues and I in the public health and infectious disease fields have been arguing for since January. Several of these points, I think, bear emphasis and should be aggressively pursued to help the U.S. emerge from the pandemic.
Support More and Faster Tests
When it comes to an infectious disease outbreak the most basic question that must be answered in any response is: "Who is infected and who is not?" Even today this simple question is not easy to answer because testing issues continue to plague us and there are voices who oppose more testing -- as if by not testing, the cases of COVID cease to exist. While testing is worlds better than it was in March – especially for hospital inpatients – it is still a process fraught with unnecessary bureaucracy and delays in the outpatient setting.
Just this past week, friends and colleagues have had to wait days upon days to get a result back, all the while having to self-quarantine pending the result. This not only leaves people in limbo, it discourages people from being tested, and renders contact tracing almost moot. A test that results in several days is almost useless to contact tracers as Bill Gates has forcefully argued.
We need more testing and more actionable rapid turn-around tests. These tests need to be deployed in healthcare facilities and beyond. Ideally, these tests should be made available for individuals to conduct on themselves at home. For some settings, such as at home, rapid antigen tests similar to those used to detect pregnancy will be suitable; for other settings, like at a doctor's office or a hospital, more elaborate PCR tests will still be key. These last have been compromised for several months due to rationing of the reagent supplies necessary to perform the test – an unacceptable state of affairs that cannot continue. Reflecting an understanding of the state of play of testing, the President-elect recently stated: "We need to increase both lab-based diagnostic testing, with results back within 24 hours or less, and faster, cheaper screening tests that you can take right at home or in school."
Roll Out Safe and Effective Vaccine(s)
Biden's plan also identifies the need to "accelerate the development of treatments and vaccines" and indeed Operation Warp Speed has been one solitary bright spot in the darkness of the failed pandemic response. It is this program that facilitated a distribution partnership with Pfizer for 100 million doses of its mRNA vaccine -- whose preliminary, and extremely positive data, was just announced today to great excitement.
Operation Warp Speed needs to be continued so that we can ensure the final development and distribution of the first-generation vaccines and treatments. When a vaccine is available, it will be a Herculean task that will span many months to actually get into the arms (twice as a 2-dose vaccine) of Americans. Vaccination may begin for healthcare workers before a change in administration, but it will continue long into 2021 and possibly longer. Vaccine distribution will be a task that demands a high degree of competence and coordination, especially with the extreme cold storage conditions needed for the vaccines.
Anticipate the Next Pandemic Now
Not only should Operation Warp Speed be supported, it needs to be expanded. For too long pandemic preparedness has been reactive and it is long past time to approach the development of medical countermeasures for pandemic threats in a proactive fashion.
What we do for other national security threats should be the paradigm for infectious disease threats that too often are subject to a mind-boggling cycle of panic and neglect. There are an estimated 200 outbreaks of viral diseases per year. Luckily and because of hard work, for many of them we have tools at our hands to control them, but for the unknown 201st virus outbreak we do not –as we've seen this year. And, the next unknown virus will likely appear soon. A new program must be constructed guaranteeing that we will never again be caught blindsided and flatfooted as we have been with the COVID-19 pandemic.
A new dedicated "Virus 201" strategy, program, and funding must be created to achieve this goal. This initiative should be a specific program focused on unknown threats that emanate from identified classes of pathogens that possess certain pandemic-causing characteristics. For example, such a program could leverage new powerful vaccine platform technologies to begin development on vaccine candidates for a variety of viral families before they emerge as full-fledged threats. Imagine how different our world would be today if this action was taken after SARS in 2003 or even MERS in 2012.
Biden should remove the handcuffs from the Centers for Disease Control and Prevention (CDC) and allow its experts to coordinate the national response and to issue guidance in the manner they were constituted to do without fear of political reprisal.
Resurrect Expertise
One of the most disheartening aspects of the pandemic has been the denigration and outright attacks on experts in infectious disease. Such disgusting attacks were not for any flaws, incompetence, or weakness but for their opposite -- strength and competence – and emanated from a desire to evade the grim reality. Such nihilism must end and indeed the Biden plan contains several crucial remedies, including the restoration of the White House National Security Council Directorate for Global Health Security and Biodefense, a crucial body of experts at the White House that the Trump administration bafflingly eliminated in 2018.
Additionally, Biden should remove the handcuffs from the Centers for Disease Control and Prevention (CDC) and allow its experts to coordinate the national response and to issue guidance in the manner they were constituted to do without fear of political reprisal.
Shore Up Hospital Capacity
For the foreseeable future, as control of the virus slips away in certain parts of the country, hospital capacity will be the paramount concern. Unlike many other industries, the healthcare sector is severely constrained in its ability to expand capacity because of regulatory and financial considerations. Hospital emergency preparedness has never been prioritized and until we can substantially curtail the spread of this virus, hospitals must remain vigilant.
We have seen how suspensions of "elective" procedures led to alarming declines in vital healthcare services that range from childhood immunization to cancer chemotherapy to psychiatric care. This cannot be allowed to happen again. Hospitals will need support in terms of staffing, alternative care sites, and personal protective equipment. Reflecting these concerns, the Biden plan outlines an approach that smartly uses the Departments of Defense and Veterans Affairs assets and medical reserve corps, coupled to the now-flourishing telemedicine innovations, to augment capacity and forestall the need for hospitals to shift to crisis standards of care.
To these five tasks, I would add a long list of subtasks that need to be executed by agencies such as the Centers for Medicare and Medicaid, the Food and Drug Administration, and many other arms of government. But, to me, these are the most crucial.
***
As COVID-19 has demonstrated, new deadly viruses can spread quickly and easily around the globe, causing significant loss of life and economic ruin. With nearly 200 epidemics occurring each year, the next fast-moving, novel infectious disease pandemic could be right around the corner.
The upcoming transition affords the opportunity to implement a new paradigm in pandemic response, biosecurity, and emerging disease response. The United States and President-elect Biden must work hard to to end this pandemic and increase the resilience of the United States to the future infectious disease threats we will surely face.
Dr. Adalja is focused on emerging infectious disease, pandemic preparedness, and biosecurity. He has served on US government panels tasked with developing guidelines for the treatment of plague, botulism, and anthrax in mass casualty settings and the system of care for infectious disease emergencies, and as an external advisor to the New York City Health and Hospital Emergency Management Highly Infectious Disease training program, as well as on a FEMA working group on nuclear disaster recovery. Dr. Adalja is an Associate Editor of the journal Health Security. He was a coeditor of the volume Global Catastrophic Biological Risks, a contributing author for the Handbook of Bioterrorism and Disaster Medicine, the Emergency Medicine CorePendium, Clinical Microbiology Made Ridiculously Simple, UpToDate's section on biological terrorism, and a NATO volume on bioterrorism. He has also published in such journals as the New England Journal of Medicine, the Journal of Infectious Diseases, Clinical Infectious Diseases, Emerging Infectious Diseases, and the Annals of Emergency Medicine. He is a board-certified physician in internal medicine, emergency medicine, infectious diseases, and critical care medicine. Follow him on Twitter: @AmeshAA

