New tools could catch disease outbreaks earlier - or predict them

A team at the Catalan Institution for Research and Advanced Studies is utilizing drones and weather stations to collect data on how mosquito breeding patterns are changing in response to climate shifts.
Every year, the villages which lie in the so-called ‘Nipah belt’— which stretches along the western border between Bangladesh and India, brace themselves for the latest outbreak. For since 1998, when Nipah virus—a form of hemorrhagic fever most common in Bangladesh—first spilled over into humans, it has been a grim annual visitor to the people of this region.
With a 70 percent fatality rate, no vaccine, and no known treatments, Nipah virus has been dubbed in the Western world as ‘the worst disease no one has ever heard of.’ Currently, outbreaks tend to be relatively contained because it is not very transmissible. The virus circulates throughout Asia in fruit eating bats, and only tends to be passed on to people who consume contaminated date palm sap, a sweet drink which is harvested across Bangladesh.
But as SARS-CoV-2 has shown the world, this can quickly change.
“Nipah virus is among what virologists call ‘the Big 10,’ along with things like Lassa fever and Crimean Congo hemorrhagic fever,” says Noam Ross, a disease ecologist at New York-based non-profit EcoHealth Alliance. “These are pretty dangerous viruses from a lethality perspective, which don’t currently have the capacity to spread into broader human populations. But that can evolve, and you could very well see a variant emerge that has human-human transmission capability.”
That’s not an overstatement. Surveys suggest that mammals harbour about 40,000 viruses, with roughly a quarter capable of infecting humans. The vast majority never get a chance to do so because we don’t encounter them, but climate change can alter that. Recent studies have found that as animals relocate to new habitats due to shifting environmental conditions, the coming decades will bring around 300,000 first encounters between species which normally don’t interact, especially in tropical Africa and southeast Asia. All these interactions will make it far more likely for hitherto unknown viruses to cross paths with humans.
That’s why for the last 16 years, EcoHealth Alliance has been conducting ongoing viral surveillance projects across Bangladesh. The goal is to understand why Nipah is so much more prevalent in the western part of the country, compared to the east, and keep a watchful eye out for new Nipah strains as well as other dangerous pathogens like Ebola.
"There are a lot of different infectious agents that are sensitive to climate change that don't have these sorts of software tools being developed for them," says Cat Lippi, medical geography researcher at the University of Florida.
Until very recently this kind of work has been hampered by the limitations of viral surveillance technology. The PREDICT project, a $200 million initiative funded by the United States Agency for International Development, which conducted surveillance across the Amazon Basin, Congo Basin and extensive parts of South and Southeast Asia, relied upon so-called nucleic acid assays which enabled scientists to search for the genetic material of viruses in animal samples.
However, the project came under criticism for being highly inefficient. “That approach requires a big sampling effort, because of the rarity of individual infections,” says Ross. “Any particular animal may be infected for a couple of weeks, maybe once or twice in its lifetime. So if you sample thousands and thousands of animals, you'll eventually get one that has an Ebola virus infection right now.”
Ross explains that there is now far more interest in serological sampling—the scientific term for the process of drawing blood for antibody testing. By searching for the presence of antibodies in the blood of humans and animals, scientists have a greater chance of detecting viruses which started circulating recently.
Despite the controversy surrounding EcoHealth Alliance’s involvement in so-called gain of function research—experiments that study whether viruses might mutate into deadlier strains—the organization’s separate efforts to stay one step ahead of pathogen evolution are key to stopping the next pandemic.
“Having really cheap and fast surveillance is really important,” says Ross. “Particularly in a place where there's persistent, low level, moderate infections that potentially have the ability to develop into more epidemic or pandemic situations. It means there’s a pathway that something more dangerous can come through."
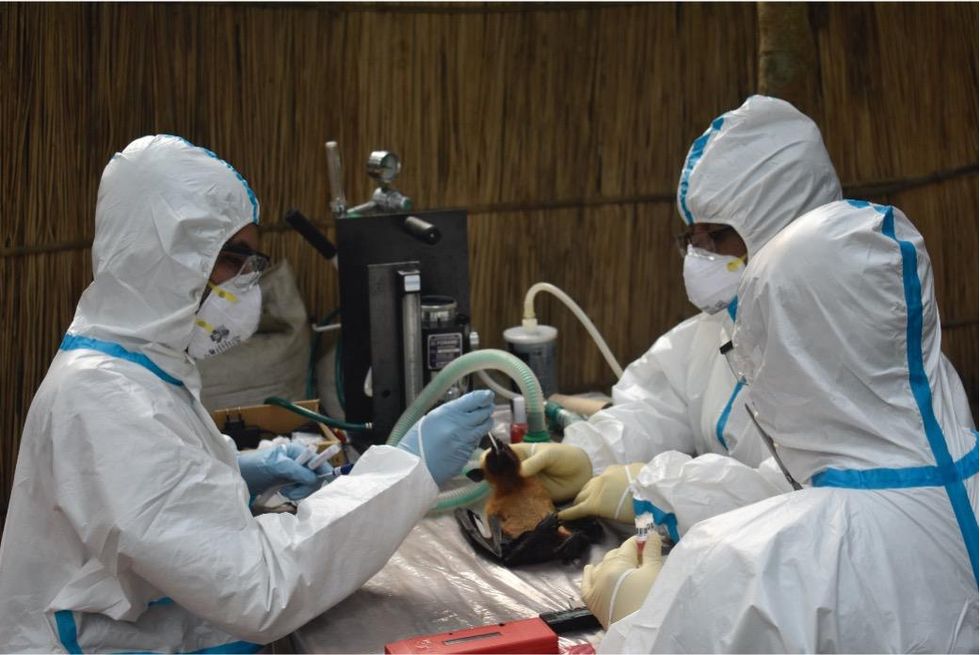
Scientists are searching for the presence of antibodies in the blood of humans and animals in hopes to detect viruses that recently started circulating.
EcoHealth Alliance
In Bangladesh, EcoHealth Alliance is attempting to do this using a newer serological technology known as a multiplex Luminex assay, which tests samples against a panel of known antibodies against many different viruses. It collects what Ross describes as a ‘footprint of information,’ which allows scientists to tell whether the sample contains the presence of a known pathogen or something completely different and needs to be investigated further.
By using this technology to sample human and animal populations across the country, they hope to gain an idea of whether there are any novel Nipah virus variants or strains from the same family, as well as other deadly viral families like Ebola.
This is just one of several novel tools being used for viral discovery in surveillance projects around the globe. Multiple research groups are taking PREDICT’s approach of looking for novel viruses in animals in various hotspots. They collect environmental DNA—mucus, faeces or shed skin left behind in soil, sediment or water—which can then be genetically sequenced.
Five years ago, this would have been a painstaking work requiring bringing collected samples back to labs. Today, thanks to the vast amounts of money spent on new technologies during COVID-19, researchers now have portable sequencing tools they can take out into the field.
Christopher Jerde, a researcher at the UC Santa Barbara Marine Science Institute, points to the Oxford Nanopore MinION sequencer as one example. “I tried one of the early versions of it four years ago, and it was miserable,” he says. “But they’ve really improved, and what we’re going to be able to do in the next five to ten years will be amazing. Instead of having to carefully transport samples back to the lab, we're going to have cigar box-shaped sequencers that we take into the field, plug into a laptop, and do the whole sequencing of an organism.”
In the past, viral surveillance has had to be very targeted and focused on known families of viruses, potentially missing new, previously unknown zoonotic pathogens. Jerde says that the rise of portable sequencers will lead to what he describes as “true surveillance.”
“Before, this was just too complex,” he says. “It had to be very focused, for example, looking for SARS-type viruses. Now we’re able to say, ‘Tell us all the viruses that are here?’ And this will give us true surveillance – we’ll be able to see the diversity of all the pathogens which are in these spots and have an understanding of which ones are coming into the population and causing damage.”
But being able to discover more viruses also comes with certain challenges. Some scientists fear that the speed of viral discovery will soon outpace the human capacity to analyze them all and assess the threat that they pose to us.
“I think we're already there,” says Jason Ladner, assistant professor at Northern Arizona University’s Pathogen and Microbiome Institute. “If you look at all the papers on the expanding RNA virus sphere, there are all of these deposited partial or complete viral sequences in groups that we just don't know anything really about yet.” Bats, for example, carry a myriad of viruses, whose ability to infect human cells we understand very poorly.
Cultivating these viruses under laboratory conditions and testing them on organoids— miniature, simplified versions of organs created from stem cells—can help with these assessments, but it is a slow and painstaking work. One hope is that in the future, machine learning could help automate this process. The new SpillOver Viral Risk Ranking platform aims to assess the risk level of a given virus based on 31 different metrics, while other computer models have tried to do the same based on the similarity of a virus’s genomic sequence to known zoonotic threats.
However, Ladner says that these types of comparisons are still overly simplistic. For one thing, scientists are still only aware of a few hundred zoonotic viruses, which is a very limited data sample for accurately assessing a novel pathogen. Instead, he says that there is a need for virologists to develop models which can determine viral compatibility with human cells, based on genomic data.
“One thing which is really useful, but can be challenging to do, is understand the cell surface receptors that a given virus might use,” he says. “Understanding whether a virus is likely to be able to use proteins on the surface of human cells to gain entry can be very informative.”
As the Earth’s climate heats up, scientists also need to better model the so-called vector borne diseases such as dengue, Zika, chikungunya and yellow fever. Transmitted by the Aedes mosquito residing in humid climates, these blights currently disproportionally affect people in low-income nations. But predictions suggest that as the planet warms and the pests find new homes, an estimated one billion people who currently don’t encounter them might be threatened by their bites by 2080. “When it comes to mosquito-borne diseases we have to worry about shifts in suitable habitat,” says Cat Lippi, a medical geography researcher at the University of Florida. “As climate patterns change on these big scales, we expect to see shifts in where people will be at risk for contracting these diseases.”
Public health practitioners and government decision-makers need tools to make climate-informed decisions about the evolving threat of different infectious diseases. Some projects are already underway. An ongoing collaboration between the Catalan Institution for Research and Advanced Studies and researchers in Brazil and Peru is utilizing drones and weather stations to collect data on how mosquitoes change their breeding patterns in response to climate shifts. This information will then be fed into computer algorithms to predict the impact of mosquito-borne illnesses on different regions.

The team at the Catalan Institution for Research and Advanced Studies is using drones and weather stations to collect data on how mosquito breeding patterns change due to climate shifts.
Gabriel Carrasco
Lippi says that similar models are urgently needed to predict how changing climate patterns affect respiratory, foodborne, waterborne and soilborne illnesses. The UK-based Wellcome Trust has allocated significant assets to fund such projects, which should allow scientists to monitor the impact of climate on a much broader range of infections. “There are a lot of different infectious agents that are sensitive to climate change that don't have these sorts of software tools being developed for them,” she says.
COVID-19’s havoc boosted funding for infectious disease research, but as its threats begin to fade from policymakers’ focus, the money may dry up. Meanwhile, scientists warn that another major infectious disease outbreak is inevitable, potentially within the next decade, so combing the planet for pathogens is vital. “Surveillance is ultimately a really boring thing that a lot of people don't want to put money into, until we have a wide scale pandemic,” Jerde says, but that vigilance is key to thwarting the next deadly horror. “It takes a lot of patience and perseverance to keep looking.”
This article originally appeared in One Health/One Planet, a single-issue magazine that explores how climate change and other environmental shifts are increasing vulnerabilities to infectious diseases by land and by sea. The magazine probes how scientists are making progress with leaders in other fields toward solutions that embrace diverse perspectives and the interconnectedness of all lifeforms and the planet.
Drugs That Trick Older People’s Bodies to Behave Younger Might Boost the Effectiveness of a COVID-19 Vaccine
Because vaccination may be less effective in older people, it is imperative to test now whether immune boosters could help the efficacy of a COVID-19 vaccine in the elderly.
In our April 23rd editorial for this magazine, we argued that addressing the COVID-19 pandemic requires that we both fight the SARS-CoV-2 virus and fortify the human hosts who are most vulnerable to it.
Two recent phase 2 studies in older adults have suggested that a new category of drugs called rapalogues can in some cases increase the immunization capacity of older adults.
Because people over 70 account for more than 80 percent of reported COVID-19 deaths globally, this means we must do everything possible to protect our elders.
A range of recent studies have suggested that systemic knobs might metaphorically be turned to slow the cellular aging process, making us better able to fight off the many diseases correlated with aging. These types of systemic changes might be used to stem the specific decline in immunity caused by aging and to increases the biological capacity of elderly people to effectively fight viral infection.
But while helping make older people more resilient in the face of a viral infection is critical, that's not the only way geroscience can help in our fight against this deadly pandemic.
As we move toward hopefully developing one or more COVID-19 vaccines, researchers must more fully appreciate the ways in which traditional vaccines can be less effective in older people than in younger ones.
Repeated studies have shown that the flu vaccine, for example, has lower efficacy in older people than in younger ones. Older people tend to develop fewer antibodies after being vaccinated because a subset of their white blood cells, called T cells, have become less responsive over time. Some inflammatory peptides that increase with aging are also preventing the action of those T cells.
This is why there's a distinct possibility that a future COVD-19 vaccine, particularly one utilizing the traditional attenuated virus approach, could be less effective in older people than in younger ones.
Given the extreme urgency of developing vaccines that work well for everyone, we need to make sure that researchers are exploring all of the ways our elders can be best protected. While generating a vaccine that works equally well for people of all ages would be ideal, we can't count on that.
One way to bridge this gap might be to trick the bodies of older people into behaving as if they are younger just at the moment what a vaccine is delivered by giving them pre-immunization boosters.
Two recent phase 2 studies in older adults have suggested that a new category of drugs called rapalogues can in some cases increase the immunization capacity of older adults. Use of the drug for a short time period before flu shot immunization increased the antibody production for the flu and resulted in a 52 percent decrease in the occurrence of severe diseases needing medical help or hospitalization. This short-term pre-immunization intervention can also decrease the severity of serious respiratory tract infections, the deadliest manifestations of COVID-19, by similar magnitude. These patients also had almost half the incidence of the non-COVID-19 coronaviruses associated with the common cold.
The fact that those people were protected by treatment before hospitalization suggests metformin may have a role in boosting the vaccination of older people.
An inexpensive generic drug called metformin similarly targets the decline in immunity and inflammation (and extends health span and lifespan) in animals and has been used for decades to protect against the flu. A recent paper from a hospital in Wuhan, China showed that mortality of elderly COVID-19 diabetic patients on metformin was 25 percent less than that of patients with diabetes but not on metformin.
Another study from the U.S. showed that COVID-19 patients on metformin had a 20 percent decrease in mortality and lower inflammation. The fact that those people were protected by treatment before hospitalization suggests metformin may have a role in boosting the vaccination of older people.
We don't yet know whether rapalogues or metformin could be used as COVID-19 immunization boosters, not least because we don't have those vaccines. But we can and should make sure that all vaccine trials including older subjects also consider offering a subset of those subjects appropriate doses of rapalogues or metformin to explore whether doing so can boost the efficacy of a given vaccine.
If we weren't in the middle of the worst pandemic in a century, we would have more time to test our vaccines slowly and sequentially. In the context of the current crisis, however, testing whether immunization boosters might increase the efficacy of potential COVID-19 vaccines for older adults is at the very least a hypothesis worth exploring.
How We Can Return to Normal Life in the COVID-19 Era
A crowded baseball stadium is the epitome of "getting back to normal."
I was asked recently when life might return to normal. The question is simple but the answer is complex, with many knowns, lots of known unknowns, and some unknown unknowns. But I'll give it my best shot.
To get the fatality rate down to flu-like levels would require that we cut Covid-19 fatalities down by a factor of 5.
Since I'm human (and thus want my life back), I might be biased toward optimism.
Here's one way to think about it: Is there another infection that causes sickness and death at levels that we tolerate? The answer, of course, is 'yes': influenza.
According to the Centers for Disease Control, from 2010 to 2019, an average of 30 million Americans had the flu each year, leading to an annual average of 37,000 deaths. This works out to an infection-fatality rate, or IFR, of 0.12 percent. We've tolerated that level of illness death from influenza for a century.
Before going on, let's get one thing out of the way: Back in March, Covid-19 wasn't, as some maintained, "like the flu," and it still isn't. Since then, the U.S. has had 3.9 million confirmed Covid-19 cases and 140,000 deaths, for an IFR of 3.6 percent. Taking all the cases — including asymptomatic patients and those with minimal symptoms who were never tested for Covid-19 — into account, the real IFR is probably 0.6 percent, or roughly 5 times that of the flu.
Nonetheless, even a partly effective vaccine, combined with moderately effective medications, could bring Covid-19 numbers down to a tolerable, flu-like, threshold. It's a goal that seems within our reach.
Chronic mask-wearing and physical distancing are not my idea of normal, nor, I would venture to guess, would most other Americans consider these desirable states in which to live. We need both now to achieve some semblance of normalcy, but they're decidedly not normal life. My notion of normal: daily life with no or minimal mask wearing, open restaurants and bars, ballparks with fans, and theaters with audiences.
My projection for when we might get there: perhaps a year from now.
To get the fatality rate down to flu-like levels would require that we cut Covid-19 fatalities down by a factor of 5, via some combination of fewer symptomatic cases and a lower chance that a symptomatic patient will go on to die. How might that happen?
First, we have to make some impact on young people – getting them to follow the public health directives at higher rates than they are currently. The main reason we need to push younger people to stay safe is that they can spread Covid-19 to vulnerable people (those who are older, with underlying health problems). But, once the most vulnerable are protected (through the deployment of some combination of effective medications and a vaccine), the fact that some young people aren't acting safely – or maybe won't take a vaccine themselves – wouldn't cause so much concern. The key is whether the people at highest risk for bad outcomes are protected.
Then there's the vaccine. The first principle: We don't need a 100 percent-effective vaccine injected into 320 million deltoid muscles (in the U.S. alone). Thank God, since it's fanciful to believe that we can have a vaccine that's 100 percent effective, universally available by next summer, and that each and every American agrees to be vaccinated.
How are we doing in our vaccine journey? We've been having some banner days lately, with recent optimistic reports from several of the vaccine companies. In one report, the leading candidate vaccine, the one effort being led by Oxford University, led to both antibodies and a cellular immune response, a very helpful belt-and-suspenders approach that increases the probability of long-lasting immunity. This good news comes on the heels of the positive news regarding the American vaccine being made by Moderna earlier in July.
While every article about vaccines sounds the obligatory cautionary notes, to date we've checked every box on the path to a safe and effective vaccine. We might not get there, but most experts are now predicting an FDA-approvable vaccine (more than 50 percent effective with no show-stopping side effects) by early 2021.
It is true that we don't know how long immunity will last, but that can be a problem to solve later. In this area, time is our friend. If we can get to an effective vaccine that lasts for a year or two, over time we should be able to discover strategies (more vaccine boosters, new and better medications) to address the possibility of waning immunity.
All things considered, I'm going to put my nickel down on the following optimistic scenario: we'll have one, and likely several, vaccines that have been proven to be more than 50 percent effective and safe by January, 2021.
If only that were the finish line.
Once we vaccinate a large fraction of high-risk patients, having a moderate number of unvaccinated people running around won't pose as much threat.
The investments in manufacturing and distribution should pay off, but it's still inconceivable that we'll be able to get vaccines to 300 million people in three to six months. For the 2009 Swine Flu, we managed to vaccinate about 1 in 4 Americans over six months.
So we'll need to prioritize. First in line will likely be the 55 million Americans over 65, and the six to eight million patient-facing healthcare workers. (How to sort priorities among people under 65 with "chronic diseases" will be a toughie.) Vaccinating 80-100 million vulnerable people, plus clinicians, might be achievable by mid-21.
If we can protect vulnerable people with an effective vaccine (with the less vulnerable waiting their turn over a subsequent 6-12 month period), that may be enough to do the trick. (Of course, vulnerable people may also be least likely to develop immunity in response to a vaccine. That could be an Achilles' heel – time will tell.)
Why might that be enough? Once we vaccinate a large fraction of high-risk patients, having a moderate number of unvaccinated people running around won't pose as much threat. Since they're at lower risk, they have a lower chance of getting sick and dying than those who received the vaccine first.
We're likely to have better meds by then, too. Since March, we've discovered two moderately effective medications for Covid-19 — remdesivir and dexamethasone. It seems likely that we'll find others by next summer, perhaps even a treatment that prevents patients from getting ill in the first place. There are many such therapies, ranging from zinc to convalescent plasma, currently being studied.
Moreover, we know that hospitals that are not overrun with Covid-19 have lower mortality rates. If we've gotten a fairly effective vaccine into most high-risk people, the hospitals are unlikely to be overwhelmed – another factor that may help lower the mortality rate to flu-like levels.
All of these factors – vaccination of most vulnerable people, one or two additional effective medications, hospitals and ICU's that aren't overwhelmed – could easily combine to bring the toll of Covid-19 down to something that resembles that of the flu. Then, we should be able to return to normal life.
Whatever the reason, if enough people refuse the vaccine, all bets are off.
What do I worry about? There's the growing anti-vaxxer movement, for one. On top of that, it seems that many Americans worry that a vaccine discovered in record speed won't be safe, or that the FDA approval process will have been corrupted by political influences. Whatever the reason, if enough people refuse the vaccine, all bets are off.
Assuming only high-risk people do get vaccinated, there will still be cases of Covid-19, maybe even mini-outbreaks, well into 2021 and likely 2022. Obviously, that's not ideal, and we should hope for a vaccine that results in the complete eradication of Covid-19. But the point is that, even with flu-like levels of illness and death, we should still be able to achieve "normal."
Hope is not a strategy, as the saying goes. But it is hope, which is more than we've had for a while.