New tools could catch disease outbreaks earlier - or predict them

A team at the Catalan Institution for Research and Advanced Studies is utilizing drones and weather stations to collect data on how mosquito breeding patterns are changing in response to climate shifts.
Every year, the villages which lie in the so-called ‘Nipah belt’— which stretches along the western border between Bangladesh and India, brace themselves for the latest outbreak. For since 1998, when Nipah virus—a form of hemorrhagic fever most common in Bangladesh—first spilled over into humans, it has been a grim annual visitor to the people of this region.
With a 70 percent fatality rate, no vaccine, and no known treatments, Nipah virus has been dubbed in the Western world as ‘the worst disease no one has ever heard of.’ Currently, outbreaks tend to be relatively contained because it is not very transmissible. The virus circulates throughout Asia in fruit eating bats, and only tends to be passed on to people who consume contaminated date palm sap, a sweet drink which is harvested across Bangladesh.
But as SARS-CoV-2 has shown the world, this can quickly change.
“Nipah virus is among what virologists call ‘the Big 10,’ along with things like Lassa fever and Crimean Congo hemorrhagic fever,” says Noam Ross, a disease ecologist at New York-based non-profit EcoHealth Alliance. “These are pretty dangerous viruses from a lethality perspective, which don’t currently have the capacity to spread into broader human populations. But that can evolve, and you could very well see a variant emerge that has human-human transmission capability.”
That’s not an overstatement. Surveys suggest that mammals harbour about 40,000 viruses, with roughly a quarter capable of infecting humans. The vast majority never get a chance to do so because we don’t encounter them, but climate change can alter that. Recent studies have found that as animals relocate to new habitats due to shifting environmental conditions, the coming decades will bring around 300,000 first encounters between species which normally don’t interact, especially in tropical Africa and southeast Asia. All these interactions will make it far more likely for hitherto unknown viruses to cross paths with humans.
That’s why for the last 16 years, EcoHealth Alliance has been conducting ongoing viral surveillance projects across Bangladesh. The goal is to understand why Nipah is so much more prevalent in the western part of the country, compared to the east, and keep a watchful eye out for new Nipah strains as well as other dangerous pathogens like Ebola.
"There are a lot of different infectious agents that are sensitive to climate change that don't have these sorts of software tools being developed for them," says Cat Lippi, medical geography researcher at the University of Florida.
Until very recently this kind of work has been hampered by the limitations of viral surveillance technology. The PREDICT project, a $200 million initiative funded by the United States Agency for International Development, which conducted surveillance across the Amazon Basin, Congo Basin and extensive parts of South and Southeast Asia, relied upon so-called nucleic acid assays which enabled scientists to search for the genetic material of viruses in animal samples.
However, the project came under criticism for being highly inefficient. “That approach requires a big sampling effort, because of the rarity of individual infections,” says Ross. “Any particular animal may be infected for a couple of weeks, maybe once or twice in its lifetime. So if you sample thousands and thousands of animals, you'll eventually get one that has an Ebola virus infection right now.”
Ross explains that there is now far more interest in serological sampling—the scientific term for the process of drawing blood for antibody testing. By searching for the presence of antibodies in the blood of humans and animals, scientists have a greater chance of detecting viruses which started circulating recently.
Despite the controversy surrounding EcoHealth Alliance’s involvement in so-called gain of function research—experiments that study whether viruses might mutate into deadlier strains—the organization’s separate efforts to stay one step ahead of pathogen evolution are key to stopping the next pandemic.
“Having really cheap and fast surveillance is really important,” says Ross. “Particularly in a place where there's persistent, low level, moderate infections that potentially have the ability to develop into more epidemic or pandemic situations. It means there’s a pathway that something more dangerous can come through."
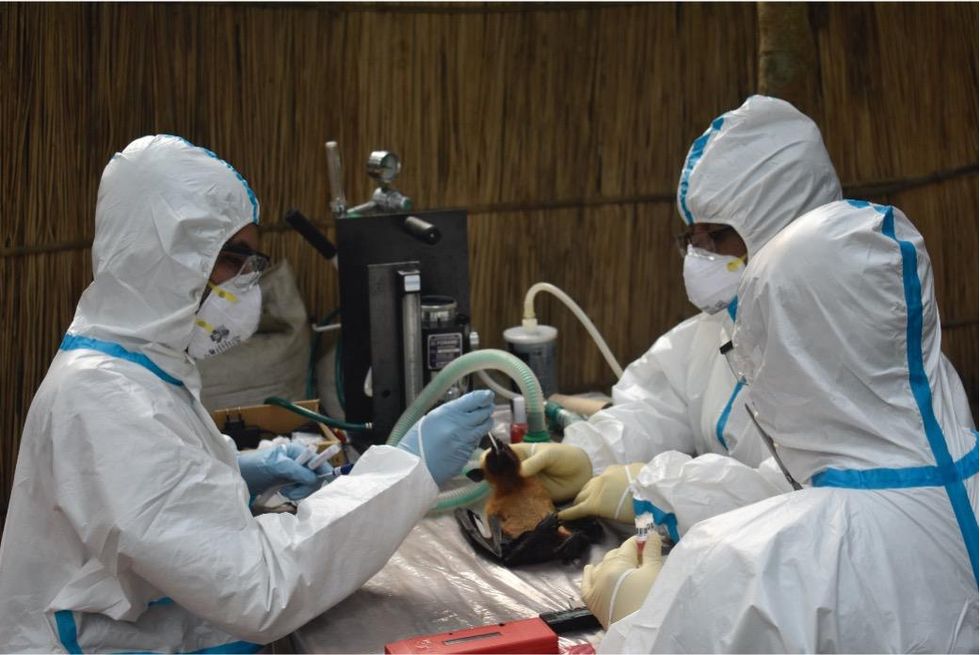
Scientists are searching for the presence of antibodies in the blood of humans and animals in hopes to detect viruses that recently started circulating.
EcoHealth Alliance
In Bangladesh, EcoHealth Alliance is attempting to do this using a newer serological technology known as a multiplex Luminex assay, which tests samples against a panel of known antibodies against many different viruses. It collects what Ross describes as a ‘footprint of information,’ which allows scientists to tell whether the sample contains the presence of a known pathogen or something completely different and needs to be investigated further.
By using this technology to sample human and animal populations across the country, they hope to gain an idea of whether there are any novel Nipah virus variants or strains from the same family, as well as other deadly viral families like Ebola.
This is just one of several novel tools being used for viral discovery in surveillance projects around the globe. Multiple research groups are taking PREDICT’s approach of looking for novel viruses in animals in various hotspots. They collect environmental DNA—mucus, faeces or shed skin left behind in soil, sediment or water—which can then be genetically sequenced.
Five years ago, this would have been a painstaking work requiring bringing collected samples back to labs. Today, thanks to the vast amounts of money spent on new technologies during COVID-19, researchers now have portable sequencing tools they can take out into the field.
Christopher Jerde, a researcher at the UC Santa Barbara Marine Science Institute, points to the Oxford Nanopore MinION sequencer as one example. “I tried one of the early versions of it four years ago, and it was miserable,” he says. “But they’ve really improved, and what we’re going to be able to do in the next five to ten years will be amazing. Instead of having to carefully transport samples back to the lab, we're going to have cigar box-shaped sequencers that we take into the field, plug into a laptop, and do the whole sequencing of an organism.”
In the past, viral surveillance has had to be very targeted and focused on known families of viruses, potentially missing new, previously unknown zoonotic pathogens. Jerde says that the rise of portable sequencers will lead to what he describes as “true surveillance.”
“Before, this was just too complex,” he says. “It had to be very focused, for example, looking for SARS-type viruses. Now we’re able to say, ‘Tell us all the viruses that are here?’ And this will give us true surveillance – we’ll be able to see the diversity of all the pathogens which are in these spots and have an understanding of which ones are coming into the population and causing damage.”
But being able to discover more viruses also comes with certain challenges. Some scientists fear that the speed of viral discovery will soon outpace the human capacity to analyze them all and assess the threat that they pose to us.
“I think we're already there,” says Jason Ladner, assistant professor at Northern Arizona University’s Pathogen and Microbiome Institute. “If you look at all the papers on the expanding RNA virus sphere, there are all of these deposited partial or complete viral sequences in groups that we just don't know anything really about yet.” Bats, for example, carry a myriad of viruses, whose ability to infect human cells we understand very poorly.
Cultivating these viruses under laboratory conditions and testing them on organoids— miniature, simplified versions of organs created from stem cells—can help with these assessments, but it is a slow and painstaking work. One hope is that in the future, machine learning could help automate this process. The new SpillOver Viral Risk Ranking platform aims to assess the risk level of a given virus based on 31 different metrics, while other computer models have tried to do the same based on the similarity of a virus’s genomic sequence to known zoonotic threats.
However, Ladner says that these types of comparisons are still overly simplistic. For one thing, scientists are still only aware of a few hundred zoonotic viruses, which is a very limited data sample for accurately assessing a novel pathogen. Instead, he says that there is a need for virologists to develop models which can determine viral compatibility with human cells, based on genomic data.
“One thing which is really useful, but can be challenging to do, is understand the cell surface receptors that a given virus might use,” he says. “Understanding whether a virus is likely to be able to use proteins on the surface of human cells to gain entry can be very informative.”
As the Earth’s climate heats up, scientists also need to better model the so-called vector borne diseases such as dengue, Zika, chikungunya and yellow fever. Transmitted by the Aedes mosquito residing in humid climates, these blights currently disproportionally affect people in low-income nations. But predictions suggest that as the planet warms and the pests find new homes, an estimated one billion people who currently don’t encounter them might be threatened by their bites by 2080. “When it comes to mosquito-borne diseases we have to worry about shifts in suitable habitat,” says Cat Lippi, a medical geography researcher at the University of Florida. “As climate patterns change on these big scales, we expect to see shifts in where people will be at risk for contracting these diseases.”
Public health practitioners and government decision-makers need tools to make climate-informed decisions about the evolving threat of different infectious diseases. Some projects are already underway. An ongoing collaboration between the Catalan Institution for Research and Advanced Studies and researchers in Brazil and Peru is utilizing drones and weather stations to collect data on how mosquitoes change their breeding patterns in response to climate shifts. This information will then be fed into computer algorithms to predict the impact of mosquito-borne illnesses on different regions.

The team at the Catalan Institution for Research and Advanced Studies is using drones and weather stations to collect data on how mosquito breeding patterns change due to climate shifts.
Gabriel Carrasco
Lippi says that similar models are urgently needed to predict how changing climate patterns affect respiratory, foodborne, waterborne and soilborne illnesses. The UK-based Wellcome Trust has allocated significant assets to fund such projects, which should allow scientists to monitor the impact of climate on a much broader range of infections. “There are a lot of different infectious agents that are sensitive to climate change that don't have these sorts of software tools being developed for them,” she says.
COVID-19’s havoc boosted funding for infectious disease research, but as its threats begin to fade from policymakers’ focus, the money may dry up. Meanwhile, scientists warn that another major infectious disease outbreak is inevitable, potentially within the next decade, so combing the planet for pathogens is vital. “Surveillance is ultimately a really boring thing that a lot of people don't want to put money into, until we have a wide scale pandemic,” Jerde says, but that vigilance is key to thwarting the next deadly horror. “It takes a lot of patience and perseverance to keep looking.”
This article originally appeared in One Health/One Planet, a single-issue magazine that explores how climate change and other environmental shifts are increasing vulnerabilities to infectious diseases by land and by sea. The magazine probes how scientists are making progress with leaders in other fields toward solutions that embrace diverse perspectives and the interconnectedness of all lifeforms and the planet.
BREAKING: The First U.S. Test to Detect If a Person Has Potential Immunity to COVID-19 Was Just Developed
Testing for immunity to COVID-19 will allow frontline health workers to safely go back to work, knowing they can't spread the disease.
While testing for COVID-19 ramps up around the country, there's another kind of testing that will prove equally important to combating the pandemic: one that can detect whether someone has already been infected.
"The idea is that this assay can be established anywhere in the world following these steps."
Why is this important? As former FDA commissioner Scott Gottlieb wrote in today's Wall Street Journal: "If a sizable portion of a local community has some protection, authorities can be more confident in relying less on invasive measures. Once deployed, serological tests are cheap, straightforward, and easy to scale."
Now, a microbiology lab at the Icahn School of Medicine at Mount Sinai, led by Dr. Florian Krammer, has just announced the development of this serological test. Leapsmag spoke with Daniel Stadlbauer, a post-doctoral fellow in the lab who helped lead the work.
Is yours the first serological test available?
They did something similar in South Korea. In the U.S., it's the first of these tests.
How close are we to rolling this test out to the public?
Last week, we started this process and we finished the protocol today. Mount Sinai is trying to roll this out in the next few days in the clinic to see which patients have been infected with coronavirus recently or have been infected at all.
The protocol we uploaded today can be used as a template for other research labs or hospitals to follow the steps we provided and they should then be able to set up the antibody test. The idea is that this assay can be established anywhere in the world following these steps.
Are there any bottlenecks to getting this rolled out – supply chain or regulation obstacles?
There are no regulations that say you can't do it. Research labs and hospitals for sure can do it. I'm not aware of supply chain issues because you need basic lab equipment and materials, but I don't think those are in short supply right now.
How does the test work?
People coming to the hospital who are suspected to have infection with coronavirus, their blood gets taken routinely. This blood can be used for our test, too. The test will tell you if this person has antibodies against coronavirus. You can also test the blood of people who are not currently sick to see if this person was infected, say, a month ago. If there are antibodies in the blood, you can say this person is probably immune to getting it again.
It will be essential workers who need to be tested first, like nurses, firefighters, and doctors. It will be great to know that they would not put themselves or others at risk by going back to work because they cannot spread the disease.
"People probably cannot get reinfected once they mount a good immune response and have good antibody levels."
How soon after infection does the test detect if you have antibodies?
Usually after 7 days of infection.
How long do the antibodies last to confer immunity?
Those studies need to be done – right now it's unclear. People probably cannot get reinfected once they mount a good immune response and have good antibody levels. How long those level last still needs to be investigated. But they won't get reinfected in the next, I would say, six months.
How accurate is the test?
Very accurate. The advantage – which is bad for us but good for the test – is that humans have no baseline immunity to this coronavirus. It means that when you have not been infected, you have pretty much no antibodies, which is why it can spread so easily. But once you have antibodies in your blood, we can detect them and it's a clear difference between antibodies or no antibodies.
Where should hospitals and labs go for more information on how to build their own tests from your work?
They should check out our lab website to find the detailed protocol to download.
If I am a person who just wants to take this test to find out if I've already been infected, what should I do?
It will be done soon in the clinical setting. I don't know yet how widely it will be available. The more research labs and hospitals that set up this testing, the more people who can be tested in the future.
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
Blood Donated from Recovered Coronavirus Patients May Soon Yield a Stopgap Treatment
Antibodies from the blood of recovered patients is being tested as a stopgap treatment.
In October 1918, Lieutenant L.W. McGuire of the United States Navy sent a report to the American Journal of Public Health detailing a promising therapy that had already saved the lives of a number of officers suffering from pneumonia complications due to the Spanish influenza outbreak.
"These antibodies then become essentially drugs."
McGuire described how transfusions of blood from recovered patients – an idea which had first been trialed during a polio epidemic in 1916 – had led to rapid recovery in a series of severe pneumonia cases at a Naval Hospital in Massachusetts. "It is believed the serum has a decided influence in shortening the course of the disease, and lowering the mortality," he wrote.
Now more than a century on, this treatment – long forgotten in the western world - is once again coming to the fore during the current COVID-19 pandemic. With fatalities continuing to rise, and no vaccine expected for many months, experts are urging medical centers across the U.S. and Europe to initiate collaborations between critical care and transfusion services to offer this as an emergency treatment for those who need it most.
As of March 20, there are more than 90,000 individuals globally who have recovered from the disease. Some scientists believe that the blood of many of these people contains high levels of neutralizing antibodies that can kill the virus.
"These antibodies then become essentially drugs," said Arturo Casadevall, professor of Molecular Microbiology & Immunology at John Hopkins Bloomberg School of Public Health, who is currently co-ordinating a clinical trial of convalescent serum for COVID-19 involving 20 institutions across the US.
"We're talking about preparing a therapy right out of the serum of those that have recovered. It could also be used in patients who are already sick, but have not progressed to respiratory failure, to treat them before they enter intensive care units. That will provide a lot of support because there's a limited number of respirators and resources."
The first conclusive data on how the blood of recovered patients can help tackle COVID-19 is set to come out of China, where it was also used as an emergency treatment during the SARS and MERS outbreaks. On February 9, a severely ill patient in Wuhan was treated with convalescent serum and since then, hospitals across China have used the therapy on a total of 245 patients, with 91 reportedly showing an improvement in symptoms.
In China alone, more than 58,000 patients have now recovered from COVID-19. Casadevall said that last week the country shipped 90 tons of serum and plasma from these patients to Italy – the center of the pandemic in Europe – for emergency use.
Some of the first people to be treated are likely to be doctors and nurses in hospitals who are most at risk of exposure.
A current challenge, however, is that the blood donation from the recovered patients must be precisely timed in order to maximize the number of antibodies a future patient receives. Doctors in China say that obtaining the necessary blood samples at the right time is one of the major barriers to applying the treatment on a larger scale.
"It's difficult to get the donations," said Dr. Yuan Shi of Chongqing Medical University. "When patients have recovered from the disease, we would like to collect their blood two to four weeks afterwards. We try our best to call back the patients, but it's sometimes difficult to get them to come back within that time period."
Because of such hurdles, Japan's largest drugmaker, Takeda Pharmaceuticals, is now working to turn neutralizing antibodies from recovered COVID-19 patients into a standardized drug product. They hope to launch a clinical trial for this in the next few months.
In the U.S., Casadevall hopes blood transfusions from recovered patients can become clinically available as a therapy within the next four weeks, once regulatory approval has been received. Some of the first people to be treated are likely to be doctors and nurses in hospitals who are most at risk of exposure, to provide a protective boost in their immunity.
"A lot of healthcare workers in the U.S. have already been asked to quarantine, and you can imagine what effect that's going to have on the healthcare system," he said. "It can't take large numbers of people staying home; there's not the capacity."
But not all medical experts are convinced it's the way to go, especially when it comes to the most severe cases of COVID-19. "There's no knowing whether that treatment would be useful or not," warned Dr. Andrew Freedman, head of Cardiff University's School of Medicine in the U.K.
"There are going to be better things available in a few months, but we are facing, 'What do you do now?'"
However, Casadevall says that the treatment is not envisioned as a panacea to treating coronavirus, but simply a temporary measure which could give doctors some options until stronger options such as vaccines or new drugs are available.
"This is a stopgap option," he said. "There are going to be better things available in a few months, but we are facing, 'What do you do now?' The only thing we can offer severely ill people at the moment is respiratory support and oxygen, and we don't have anything to prevent those exposed from going on and getting ill."