New tools could catch disease outbreaks earlier - or predict them

A team at the Catalan Institution for Research and Advanced Studies is utilizing drones and weather stations to collect data on how mosquito breeding patterns are changing in response to climate shifts.
Every year, the villages which lie in the so-called ‘Nipah belt’— which stretches along the western border between Bangladesh and India, brace themselves for the latest outbreak. For since 1998, when Nipah virus—a form of hemorrhagic fever most common in Bangladesh—first spilled over into humans, it has been a grim annual visitor to the people of this region.
With a 70 percent fatality rate, no vaccine, and no known treatments, Nipah virus has been dubbed in the Western world as ‘the worst disease no one has ever heard of.’ Currently, outbreaks tend to be relatively contained because it is not very transmissible. The virus circulates throughout Asia in fruit eating bats, and only tends to be passed on to people who consume contaminated date palm sap, a sweet drink which is harvested across Bangladesh.
But as SARS-CoV-2 has shown the world, this can quickly change.
“Nipah virus is among what virologists call ‘the Big 10,’ along with things like Lassa fever and Crimean Congo hemorrhagic fever,” says Noam Ross, a disease ecologist at New York-based non-profit EcoHealth Alliance. “These are pretty dangerous viruses from a lethality perspective, which don’t currently have the capacity to spread into broader human populations. But that can evolve, and you could very well see a variant emerge that has human-human transmission capability.”
That’s not an overstatement. Surveys suggest that mammals harbour about 40,000 viruses, with roughly a quarter capable of infecting humans. The vast majority never get a chance to do so because we don’t encounter them, but climate change can alter that. Recent studies have found that as animals relocate to new habitats due to shifting environmental conditions, the coming decades will bring around 300,000 first encounters between species which normally don’t interact, especially in tropical Africa and southeast Asia. All these interactions will make it far more likely for hitherto unknown viruses to cross paths with humans.
That’s why for the last 16 years, EcoHealth Alliance has been conducting ongoing viral surveillance projects across Bangladesh. The goal is to understand why Nipah is so much more prevalent in the western part of the country, compared to the east, and keep a watchful eye out for new Nipah strains as well as other dangerous pathogens like Ebola.
"There are a lot of different infectious agents that are sensitive to climate change that don't have these sorts of software tools being developed for them," says Cat Lippi, medical geography researcher at the University of Florida.
Until very recently this kind of work has been hampered by the limitations of viral surveillance technology. The PREDICT project, a $200 million initiative funded by the United States Agency for International Development, which conducted surveillance across the Amazon Basin, Congo Basin and extensive parts of South and Southeast Asia, relied upon so-called nucleic acid assays which enabled scientists to search for the genetic material of viruses in animal samples.
However, the project came under criticism for being highly inefficient. “That approach requires a big sampling effort, because of the rarity of individual infections,” says Ross. “Any particular animal may be infected for a couple of weeks, maybe once or twice in its lifetime. So if you sample thousands and thousands of animals, you'll eventually get one that has an Ebola virus infection right now.”
Ross explains that there is now far more interest in serological sampling—the scientific term for the process of drawing blood for antibody testing. By searching for the presence of antibodies in the blood of humans and animals, scientists have a greater chance of detecting viruses which started circulating recently.
Despite the controversy surrounding EcoHealth Alliance’s involvement in so-called gain of function research—experiments that study whether viruses might mutate into deadlier strains—the organization’s separate efforts to stay one step ahead of pathogen evolution are key to stopping the next pandemic.
“Having really cheap and fast surveillance is really important,” says Ross. “Particularly in a place where there's persistent, low level, moderate infections that potentially have the ability to develop into more epidemic or pandemic situations. It means there’s a pathway that something more dangerous can come through."
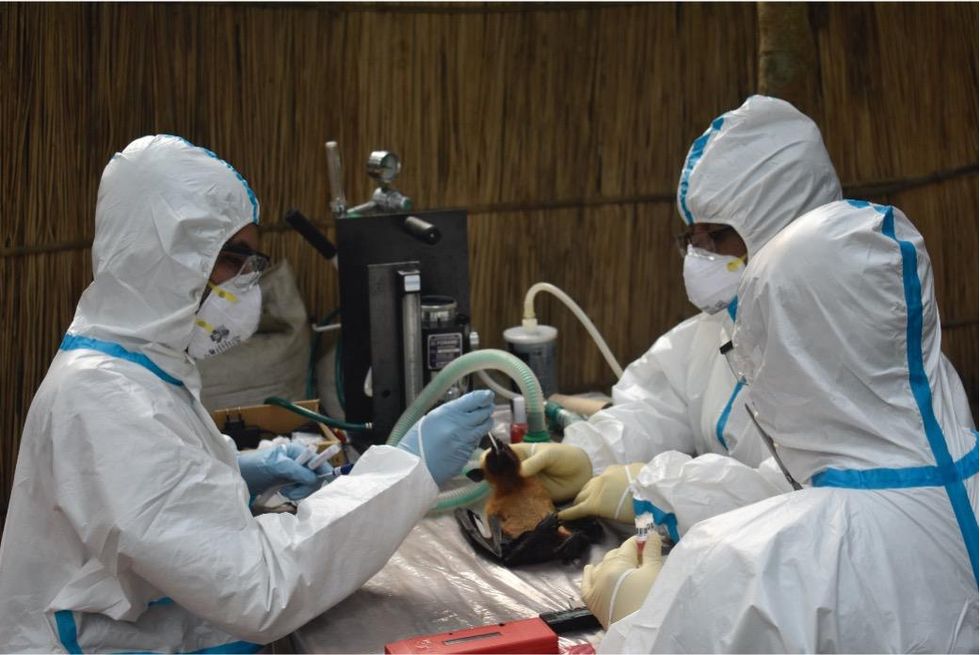
Scientists are searching for the presence of antibodies in the blood of humans and animals in hopes to detect viruses that recently started circulating.
EcoHealth Alliance
In Bangladesh, EcoHealth Alliance is attempting to do this using a newer serological technology known as a multiplex Luminex assay, which tests samples against a panel of known antibodies against many different viruses. It collects what Ross describes as a ‘footprint of information,’ which allows scientists to tell whether the sample contains the presence of a known pathogen or something completely different and needs to be investigated further.
By using this technology to sample human and animal populations across the country, they hope to gain an idea of whether there are any novel Nipah virus variants or strains from the same family, as well as other deadly viral families like Ebola.
This is just one of several novel tools being used for viral discovery in surveillance projects around the globe. Multiple research groups are taking PREDICT’s approach of looking for novel viruses in animals in various hotspots. They collect environmental DNA—mucus, faeces or shed skin left behind in soil, sediment or water—which can then be genetically sequenced.
Five years ago, this would have been a painstaking work requiring bringing collected samples back to labs. Today, thanks to the vast amounts of money spent on new technologies during COVID-19, researchers now have portable sequencing tools they can take out into the field.
Christopher Jerde, a researcher at the UC Santa Barbara Marine Science Institute, points to the Oxford Nanopore MinION sequencer as one example. “I tried one of the early versions of it four years ago, and it was miserable,” he says. “But they’ve really improved, and what we’re going to be able to do in the next five to ten years will be amazing. Instead of having to carefully transport samples back to the lab, we're going to have cigar box-shaped sequencers that we take into the field, plug into a laptop, and do the whole sequencing of an organism.”
In the past, viral surveillance has had to be very targeted and focused on known families of viruses, potentially missing new, previously unknown zoonotic pathogens. Jerde says that the rise of portable sequencers will lead to what he describes as “true surveillance.”
“Before, this was just too complex,” he says. “It had to be very focused, for example, looking for SARS-type viruses. Now we’re able to say, ‘Tell us all the viruses that are here?’ And this will give us true surveillance – we’ll be able to see the diversity of all the pathogens which are in these spots and have an understanding of which ones are coming into the population and causing damage.”
But being able to discover more viruses also comes with certain challenges. Some scientists fear that the speed of viral discovery will soon outpace the human capacity to analyze them all and assess the threat that they pose to us.
“I think we're already there,” says Jason Ladner, assistant professor at Northern Arizona University’s Pathogen and Microbiome Institute. “If you look at all the papers on the expanding RNA virus sphere, there are all of these deposited partial or complete viral sequences in groups that we just don't know anything really about yet.” Bats, for example, carry a myriad of viruses, whose ability to infect human cells we understand very poorly.
Cultivating these viruses under laboratory conditions and testing them on organoids— miniature, simplified versions of organs created from stem cells—can help with these assessments, but it is a slow and painstaking work. One hope is that in the future, machine learning could help automate this process. The new SpillOver Viral Risk Ranking platform aims to assess the risk level of a given virus based on 31 different metrics, while other computer models have tried to do the same based on the similarity of a virus’s genomic sequence to known zoonotic threats.
However, Ladner says that these types of comparisons are still overly simplistic. For one thing, scientists are still only aware of a few hundred zoonotic viruses, which is a very limited data sample for accurately assessing a novel pathogen. Instead, he says that there is a need for virologists to develop models which can determine viral compatibility with human cells, based on genomic data.
“One thing which is really useful, but can be challenging to do, is understand the cell surface receptors that a given virus might use,” he says. “Understanding whether a virus is likely to be able to use proteins on the surface of human cells to gain entry can be very informative.”
As the Earth’s climate heats up, scientists also need to better model the so-called vector borne diseases such as dengue, Zika, chikungunya and yellow fever. Transmitted by the Aedes mosquito residing in humid climates, these blights currently disproportionally affect people in low-income nations. But predictions suggest that as the planet warms and the pests find new homes, an estimated one billion people who currently don’t encounter them might be threatened by their bites by 2080. “When it comes to mosquito-borne diseases we have to worry about shifts in suitable habitat,” says Cat Lippi, a medical geography researcher at the University of Florida. “As climate patterns change on these big scales, we expect to see shifts in where people will be at risk for contracting these diseases.”
Public health practitioners and government decision-makers need tools to make climate-informed decisions about the evolving threat of different infectious diseases. Some projects are already underway. An ongoing collaboration between the Catalan Institution for Research and Advanced Studies and researchers in Brazil and Peru is utilizing drones and weather stations to collect data on how mosquitoes change their breeding patterns in response to climate shifts. This information will then be fed into computer algorithms to predict the impact of mosquito-borne illnesses on different regions.

The team at the Catalan Institution for Research and Advanced Studies is using drones and weather stations to collect data on how mosquito breeding patterns change due to climate shifts.
Gabriel Carrasco
Lippi says that similar models are urgently needed to predict how changing climate patterns affect respiratory, foodborne, waterborne and soilborne illnesses. The UK-based Wellcome Trust has allocated significant assets to fund such projects, which should allow scientists to monitor the impact of climate on a much broader range of infections. “There are a lot of different infectious agents that are sensitive to climate change that don't have these sorts of software tools being developed for them,” she says.
COVID-19’s havoc boosted funding for infectious disease research, but as its threats begin to fade from policymakers’ focus, the money may dry up. Meanwhile, scientists warn that another major infectious disease outbreak is inevitable, potentially within the next decade, so combing the planet for pathogens is vital. “Surveillance is ultimately a really boring thing that a lot of people don't want to put money into, until we have a wide scale pandemic,” Jerde says, but that vigilance is key to thwarting the next deadly horror. “It takes a lot of patience and perseverance to keep looking.”
This article originally appeared in One Health/One Planet, a single-issue magazine that explores how climate change and other environmental shifts are increasing vulnerabilities to infectious diseases by land and by sea. The magazine probes how scientists are making progress with leaders in other fields toward solutions that embrace diverse perspectives and the interconnectedness of all lifeforms and the planet.
A pregnant woman is uncertain which medicine is safe to take.
Sarah Mancoll was 22 years old when she noticed a bald spot on the back of her head. A dermatologist confirmed that it was alopecia aerata, an autoimmune disorder that causes hair loss.
Of 213 new drugs approved from 2003 to 2012, only five percent included any data from pregnant women.
She successfully treated the condition with corticosteroid shots for nearly 10 years. Then Mancoll and her husband began thinking about starting a family. Would the shots be safe for her while pregnant? For the fetus? What about breastfeeding?
Mancoll consulted her primary care physician, her dermatologist, even a pediatrician. Without clinical data, no one could give her a definitive answer, so she stopped treatment to be "on the safe side." By the time her son was born, she'd lost at least half her hair. She returned to her Washington, D.C., public policy job two months later entirely bald—and without either eyebrows or eyelashes.
After having two more children in quick succession, Mancoll recently resumed the shots but didn't forget her experience. Today, she is an advocate for including more pregnant and lactating women in clinical studies so they can have more information about therapies than she did.
"I live a very privileged life, and I'll do just fine with or without hair, but it's not just about me," Mancoll said. "It's about a huge population of women who are being disenfranchised…They're invisible."
About 4 million women give birth each year in the United States, and many face medical conditions, from hypertension and diabetes to psychiatric disorders. A 2011 study showed that most women reported taking at least one medication while pregnant between 1976 and 2008. But for decades, pregnant and lactating women have been largely excluded from clinical drug studies that rigorously test medications for safety and effectiveness.
An estimated 98 percent of government-approved drug treatments between 2000 and 2010 had insufficient data to determine risk to the fetus, and close to 75 percent had no human pregnancy data at all. All told, of 213 new pharmaceuticals approved from 2003 to 2012, only five percent included any data from pregnant women.
But recent developments suggest that could be changing. Amid widespread concerns about increased maternal mortality rates, women's health advocates, physicians, and researchers are sensing and encouraging a cultural shift toward protecting women through responsible research instead of from research.
"The question is not whether to do research with pregnant women, but how," Anne Drapkin Lyerly, professor and associate director of the Center for Bioethics at the University of North Carolina at Chapel Hill, wrote last year in an op-ed. "These advances are essential. It is well past time—and it is morally imperative—for research to benefit pregnant women."
"In excluding pregnant women from drug trials to protect them from experimentation, we subject them to uncontrolled experimentation."
To that end, the American College of Obstetricians and Gynecologists' Committee on Ethics acknowledged that research trials need to be better designed so they don't "inappropriately constrain the reproductive choices of study participants or unnecessarily exclude pregnant women." A federal task force also called for significantly expanded research and the removal of regulatory barriers that make it difficult for pregnant and lactating women to participate in research.
Several months ago, a government change to a regulation known as the Common Rule took effect, removing pregnant women as a "vulnerable population" in need of special protections -- a designation that had made it more difficult to enroll them in clinical drug studies. And just last week, the U.S. Food and Drug Administration (FDA) issued new draft guidances for industry on when and how to include pregnant and lactating women in clinical trials.
Inclusion is better than the absence of data on their treatment, said Catherine Spong, former chair of the federal task force.
"It's a paradox," said Spong, professor of obstetrics and gynecology and chief of maternal fetal medicine at University of Texas Southwestern Medical Center. "There is a desire to protect women and fetuses from harm, which is translated to a reluctance to include them in research. By excluding them, the evidence for their care is limited."
Jacqueline Wolf, a professor of the history of medicine at Ohio University, agreed.
"In excluding pregnant women from drug trials to protect them from experimentation, we subject them to uncontrolled experimentation," she said. "We give them the medication without doing any research, and that's dangerous."
Women, of course, don't stop getting sick or having chronic medical conditions just because they are pregnant or breastfeeding, and conditions during pregnancy can affect a baby's health later in life. Evidence-based data is important for other reasons, too.
Pregnancy can dramatically change a woman's physiology, affecting how drugs act on her body and how her body acts or reacts to drugs. For instance, pregnant bodies can more quickly clear out medications such as glyburide, used during diabetes in pregnancy to stabilize high blood-sugar levels, which can be toxic to the fetus and harmful to women. That means a regular dose of the drug may not be enough to control blood sugar and prevent poor outcomes.
Pregnant patients also may be reluctant to take needed drugs for underlying conditions (and doctors may be hesitant to prescribe them), which in turn can cause more harm to the woman and fetus than had they been treated. For example, women who have severe asthma attacks while pregnant are at a higher risk of having low-birthweight babies, and pregnant women with uncontrolled diabetes in early pregnancy have more than four times the risk of birth defects.
Current clinical trials involving pregnant women are assessing treatments for obstructive sleep apnea, postpartum hemorrhage, lupus, and diabetes.
For Kate O'Brien, taking medication during her pregnancy was a matter of life and death. A freelance video producer who lives in New Jersey, O'Brien was diagnosed with tuberculosis in 2015 after she became pregnant with her second child, a boy. Even as she signed hospital consent forms, she had no idea if the treatment would harm him.
"It's a really awful experience," said O'Brien, who now is active with We are TB, an advocacy and support network. "All they had to tell me about the medication was just that women have been taking it for a really long time all over the world. That was the best they could do."
More and more doctors, researchers and women's health organizations and advocates are calling that unacceptable.
By indicating that filling current knowledge gaps is "a critical public health need," the FDA is signaling its support for advancing research with pregnant women, said Lyerly, also co-founder of the Second Wave Initiative, which promotes fair representation of the health interests of pregnant women in biomedical research and policies. "It's a very important shift."
Research with pregnant women can be done ethically, Lyerly said, whether by systematically collecting data from those already taking medications or enrolling pregnant women in studies of drugs or vaccines in development.
Current clinical trials involving pregnant women are assessing treatments for obstructive sleep apnea, postpartum hemorrhage, lupus, and diabetes. Notable trials in development target malaria and HIV prevention in pregnancy.
"It clearly is doable to do this research, and test trials are important to provide evidence for treatment," Spong said. "If we don't have that evidence, we aren't making the best educated decisions for women."
Study of the DNA double helix will lead to transformative medical care and increasingly urgent questions about how to responsibly handle genetic engineering technology.
The news last November that a rogue Chinese scientist had genetically altered the embryos of a pair of Chinese twins shocked the world. But although this use of advanced technology to change the human gene pool was premature, it was a harbinger of how genetic science will alter our healthcare, the way we make babies, the nature of the babies we make, and, ultimately, our sense of who and what we are as a species.
The healthcare applications of the genetics revolution are merely stations along the way to the ultimate destination.
But while the genetics revolution has already begun, we aren't prepared to handle these Promethean technologies responsibly.
By identifying the structure of DNA in the 1950s, Watson, Crick, Wilkins, and Franklin showed that the book of life was written in the DNA double helix. When the human genome project was completed in 2003, we saw how this book of human life could be transcribed. Painstaking research paired with advanced computational algorithms then showed what increasing numbers of genes do and how the genetic book of life can be read.
Now, with the advent of precision gene editing tools like CRISPR, we are seeing that the book of life -- and all biology -- can be re-written. Biology is being recognized as another form of readable, writable, and hackable information technology with we humans as the coders.
The impact of this transformation is being first experienced in our healthcare. Gene therapies including those extracting, re-engineering, then reintroducing a person's own cells enhanced into cancer-fighting supercells are already performing miracles in clinical trials. Thousands of applications have already been submitted to regulators across the globe for trials using gene therapies to address a host of other diseases.
Recently, the first gene editing of cells inside a person's body was deployed to treat the genetically relatively simple metabolic disorder Hunter syndrome, with many more applications to come. These new approaches are only the very first steps in our shift from the current system of generalized medicine based on population averages to precision medicine based on each patient's individual biology to predictive medicine based on AI-generated estimations of a person's future health state.

Jamie Metzl's groundbreaking new book, Hacking Darwin: Genetic Engineering and the Future of Humanity, explores how the genetic revolution is transforming our healthcare, the way we make babies, and the nature of and babies we make, what this means for each of us, and what we must all do now to prepare for what's coming.
This shift in our healthcare will ensure that millions and then billions of people will have their genomes sequenced as the foundation of their treatment. Big data analytics will then be used to compare at scale people's genotypes (what their genes say) to their phenotypes (how those genes are expressed over the course of their lives).
These massive datasets of genetic and life information will then make it possible to go far beyond the simple genetic analysis of today and to understand far more complex human diseases and traits influenced by hundreds or thousands of genes. Our understanding of this complex genetic system within the vaster ecosystem of our bodies and the environment around us will transform healthcare for the better and help us cure terrible diseases that have plagued our ancestors for millennia.
But as revolutionary as this challenge will be for medicine, the healthcare applications of the genetics revolution are merely stations along the way to the ultimate destination – a deep and fundamental transformation of our evolutionary trajectory as a species.
A first inkling of where we are heading can be seen in the direct-to-consumer genetic testing industry. Many people around the world have now sent their cheek swabs to companies like 23andMe for analysis. The information that comes back can tell people a lot about relatively simple genetic traits like carrier status for single gene mutation diseases, eye color, or whether they hate the taste of cilantro, but the information about complex traits like athletic predisposition, intelligence, or personality style today being shared by some of these companies is wildly misleading.
This will not always be the case. As the genetic and health data pools grow, analysis of large numbers of sequenced genomes will make it possible to apply big data analytics to predict some very complex genetic disease risks and the genetic components of traits like height, IQ, temperament, and personality style with increasing accuracy. This process, called "polygenic scoring," is already being offered in beta stage by a few companies and will become an ever bigger part of our lives going forward.
The most profound application of all this will be in our baby-making. Before making a decision about which of the fertilized eggs to implant, women undergoing in vitro fertilization can today elect to have a small number of cells extracted from their pre-implanted embryos and sequenced. With current technology, this can be used to screen for single-gene mutation diseases and other relatively simple disorders. Polygenic scoring, however, will soon make it possible to screen these early stage pre-implanted embryos to assess their risk of complex genetic diseases and even to make predictions about the heritable parts of complex human traits. The most intimate elements of being human will start feeling like high-pressure choices needing to be made by parents.
The limit of our imagination will become the most significant barrier to our recasting biology.
Adult stem cell technologies will then likely make it possible to generate hundreds or thousands of a woman's own eggs from her blood sample or skin graft. This would blow open the doors of reproductive possibility and allow parents to choose embryos with exceptional potential capabilities from a much larger set of options.
The complexity of human biology will place some limits to the extent of possible gene edits that might be made to these embryos, but all of biology, including our own, is extremely flexible. How else could all the diversity of life have emerged from a single cell nearly four billion years ago? The limit of our imagination will become the most significant barrier to our recasting biology.
But while we humans are gaining the powers of the gods, we aren't at all ready to use them.
The same tools that will help cure our worst afflictions, save our children, help us live longer, healthier, more robust lives will also open the door to potential abuses. Prospective parents with the best of intentions or governments with lax regulatory structures or aggressive ideas of how population-wide genetic engineering might be used to enhance national competitiveness or achieve some other goal could propel us into a genetic arms race that could undermine our essential diversity, dangerously divide societies, lead to dangerous, destabilizing, and potentially even deadly conflicts between us, and threaten our very humanity.
But while the advance of genetic technologies is inevitable, how it plays out is anything but. If we don't want the genetic revolution to undermine our species or lead to grave conflicts between genetic haves and have nots or between societies opting in and those opting out, now is the time when we need to make smart decisions based on our individual and collective best values. Although the technology driving the genetic revolution is new, the value systems we will need to optimize the benefits and minimize the harms of this massive transformation are ones we have been developing for thousands of years.
And while some very smart and well-intentioned scientists have been meeting to explore what comes next, it won't be enough for a few of even our wisest prophets to make decisions about the future of our species that will impact everyone. We'll also need smart regulations on both the national and international levels.
Every country will need to have its own regulatory guidelines for human genetic engineering based on both international best practices and the country's unique traditions and values. Because we are all one species, however, we will also ultimately need to develop guidelines that can apply to all of us.
As a first step toward making this possible, we must urgently launch a global, species-wide education effort and inclusive dialogue on the future of human genetic engineering that can eventually inform global norms that will need to underpin international regulations. This process will not be easy, but the alternative of an unregulated genetic arms race would be far worse.
The overlapping genomics and AI revolutions may seem like distant science fiction but are closer than you think. Far sooner than most people recognize, the inherent benefits of these technologies and competition between us will spark rapid adoption. Before that spark ignites, we have a brief moment to come together as a species like we never have before to articulate and translate into action the future we jointly envision. The north star of our best shared values can help us navigate the almost unimaginable opportunities and very real challenges that lie ahead.

