Trading syphilis for malaria: How doctors treated one deadly disease by infecting patients with another

In the 1920s, doctors induced a high fever in patients - so called "fever therapy" - as a way to help them recover from syphilis, though it involved ethical problems.
If you had lived one hundred years ago, syphilis – a bacterial infection spread by sexual contact – would likely have been one of your worst nightmares. Even though syphilis still exists, it can now be detected early and cured quickly with a course of antibiotics. Back then, however, before antibiotics and without an easy way to detect the disease, syphilis was very often a death sentence.
To understand how feared syphilis once was, it’s important to understand exactly what it does if it’s allowed to progress: the infections start off as small, painless sores or even a single sore near the vagina, penis, anus, or mouth. The sores disappear around three to six weeks after the initial infection – but untreated, syphilis moves into a secondary stage, often presenting as a mild rash in various areas of the body (such as the palms of a person’s hands) or through other minor symptoms. The disease progresses from there, often quietly and without noticeable symptoms, sometimes for decades before it reaches its final stages, where it can cause blindness, organ damage, and even dementia. Research indicates, in fact, that as much as 10 percent of psychiatric admissions in the early 20th century were due to dementia caused by syphilis, also known as neurosyphilis.
Like any bacterial disease, syphilis can affect kids, too. Though it’s spread primarily through sexual contact, it can also be transmitted from mother to child during birth, causing lifelong disability.
The poet-physician Aldabert Bettman, who wrote fictionalized poems based on his experiences as a doctor in the 1930s, described the effect syphilis could have on an infant in his poem Daniel Healy:
I always got away clean
when I went out
With the boys.
The night before
I was married
I went out,—But was not so fortunate;
And I infected
My bride.
When little Daniel
Was born
His eyes discharged;
And I dared not tell
That because
I had seen too much
Little Daniel sees not at all
Given the horrors of untreated syphilis, it’s maybe not surprising that people would go to extremes to try and treat it. One of the earliest remedies for syphilis, dating back to 15th century Naples, was using mercury – either rubbing it on the skin where blisters appeared, or breathing it in as a vapor. (Not surprisingly, many people who underwent this type of “treatment” died of mercury poisoning.)
Other primitive treatments included using tinctures made of a flowering plant called guaiacum, as well as inducing “sweat baths” to eliminate the syphilitic toxins. In 1910, an arsenic-based drug called Salvarsan hit the market and was hailed as a “magic bullet” for its ability to target and destroy the syphilis-causing bacteria without harming the patient. However, while Salvarsan was effective in treating early-stage syphilis, it was largely ineffective by the time the infection progressed beyond the second stage. Tens of thousands of people each year continued to die of syphilis or were otherwise shipped off to psychiatric wards due to neurosyphilis.
It was in one of these psychiatric units in the early 20th century that Dr. Julius Wagner-Juaregg got the idea for a potential cure.
Wagner-Juaregg was an Austrian-born physician trained in “experimental pathology” at the University of Vienna. Wagner-Juaregg started his medical career conducting lab experiments on animals and then moved on to work at different psychiatric clinics in Vienna, despite having no training in psychiatry or neurology.
Wagner-Juaregg’s work was controversial to say the least. At the time, medicine – particularly psychiatric medicine – did not have anywhere near the same rigorous ethical standards that doctors, researchers, and other scientists are bound to today. Wagner-Juaregg would devise wild theories about the cause of their psychiatric ailments and then perform experimental procedures in an attempt to cure them. (As just one example, Wagner-Juaregg would sterilize his adolescent male patients, thinking “excessive masturbation” was the cause of their schizophrenia.)
But sometimes these wild theories paid off. In 1883, during his residency, Wagner-Juaregg noted that a female patient with mental illness who had contracted a skin infection and suffered a high fever experienced a sudden (and seemingly miraculous) remission from her psychosis symptoms after the fever had cleared. Wagner-Juaregg theorized that inducing a high fever in his patients with neurosyphilis could help them recover as well.
Eventually, Wagner-Juaregg was able to put his theory to the test. Around 1890, Wagner-Juaregg got his hands on something called tuberculin, a therapeutic treatment created by the German microbiologist Robert Koch in order to cure tuberculosis. Tuberculin would later turn out to be completely ineffective for treating tuberculosis, often creating severe immune responses in patients – but for a short time, Wagner-Juaregg had some success in using tuberculin to help his dementia patients. Giving his patients tuberculin resulted in a high fever – and after completing the treatment, Wagner-Jauregg reported that his patient’s dementia was completely halted. The success was short-lived, however: Wagner-Juaregg eventually had to discontinue tuberculin as a treatment, as it began to be considered too toxic.
By 1917, Wagner-Juaregg’s theory about syphilis and fevers was becoming more credible – and one day a new opportunity presented itself when a wounded soldier, stricken with malaria and a related fever, was accidentally admitted to his psychiatric unit.
When his findings were published in 1918, Wagner-Juaregg’s so-called “fever therapy” swept the globe.
What Wagner-Juaregg did next was ethically deplorable by any standard: Before he allowed the soldier any quinine (the standard treatment for malaria at the time), Wagner-Juaregg took a small sample of the soldier’s blood and inoculated three syphilis patients with the sample, rubbing the blood on their open syphilitic blisters.
It’s unclear how well the malaria treatment worked for those three specific patients – but Wagner-Juaregg’s records show that in the span of one year, he inoculated a total of nine patients with malaria, for the sole purpose of inducing fevers, and six of them made a full recovery. Wagner-Juaregg’s treatment was so successful, in fact, that one of his inoculated patients, an actor who was unable to work due to his dementia, was eventually able to find work again and return to the stage. Two additional patients – a military officer and a clerk – recovered from their once-terminal illnesses and returned to their former careers as well.
When his findings were published in 1918, Wagner-Juaregg’s so-called “fever therapy” swept the globe. The treatment was hailed as a breakthrough – but it still had risks. Malaria itself had a mortality rate of about 15 percent at the time. Many people considered that to be a gamble worth taking, compared to dying a painful, protracted death from syphilis.
Malaria could also be effectively treated much of the time with quinine, whereas other fever-causing illnesses were not so easily treated. Triggering a fever by way of malaria specifically, therefore, became the standard of care.
Tens of thousands of people with syphilitic dementia would go on to be treated with fever therapy until the early 1940s, when a combination of Salvarsan and penicillin caused syphilis infections to decline. Eventually, neurosyphilis became rare, and then nearly unheard of.
Despite his contributions to medicine, it’s important to note that Wagner-Juaregg was most definitely not a person to idolize. In fact, he was an outspoken anti-Semite and proponent of eugenics, arguing that Jews were more prone to mental illness and that people who were mentally ill should be forcibly sterilized. (Wagner-Juaregg later became a Nazi sympathizer during Hitler’s rise to power even though, bizarrely, his first wife was Jewish.) Another problematic issue was that his fever therapy involved experimental treatments on many who, due to their cognitive issues, could not give informed consent.
Lack of consent was also a fundamental problem with the syphilis study at Tuskegee, appalling research that began just 14 years after Wagner-Juaregg published his “fever therapy” findings.
Still, despite his outrageous views, Wagner-Juaregg was awarded the Nobel Prize in Medicine or Physiology in 1927 – and despite some egregious human rights abuses, the miraculous “fever therapy” was partly responsible for taming one of the deadliest plagues in human history.
Researchers advance drugs that treat pain without addiction
New therapies are using creative approaches that target the body’s sensory neurons, which send pain signals to the brain.
Opioids are one of the most common ways to treat pain. They can be effective but are also highly addictive, an issue that has fueled the ongoing opioid crisis. In 2020, an estimated 2.3 million Americans were dependent on prescription opioids.
Opioids bind to receptors at the end of nerve cells in the brain and body to prevent pain signals. In the process, they trigger endorphins, so the brain constantly craves more. There is a huge risk of addiction in patients using opioids for chronic long-term pain. Even patients using the drugs for acute short-term pain can become dependent on them.
Scientists have been looking for non-addictive drugs to target pain for over 30 years, but their attempts have been largely ineffective. “We desperately need alternatives for pain management,” says Stephen E. Nadeau, a professor of neurology at the University of Florida.
A “dimmer switch” for pain
Paul Blum is a professor of biological sciences at the University of Nebraska. He and his team at Neurocarrus have created a drug called N-001 for acute short-term pain. N-001 is made up of specially engineered bacterial proteins that target the body’s sensory neurons, which send pain signals to the brain. The proteins in N-001 turn down pain signals, but they’re too large to cross the blood-brain barrier, so they don’t trigger the release of endorphins. There is no chance of addiction.
When sensory neurons detect pain, they become overactive and send pain signals to the brain. “We wanted a way to tone down sensory neurons but not turn them off completely,” Blum reveals. The proteins in N-001 act “like a dimmer switch, and that's key because pain is sensation overstimulated.”
Blum spent six years developing the drug. He finally managed to identify two proteins that form what’s called a C2C complex that changes the structure of a subunit of axons, the parts of neurons that transmit electrical signals of pain. Changing the structure reduces pain signaling.
“It will be a long path to get to a successful clinical trial in humans," says Stephen E. Nadeau, professor of neurology at the University of Florida. "But it presents a very novel approach to pain reduction.”
Blum is currently focusing on pain after knee and ankle surgery. Typically, patients are treated with anesthetics for a short time after surgery. But anesthetics usually only last for 4 to 6 hours, and long-term use is toxic. For some, the pain subsides. Others continue to suffer after the anesthetics have worn off and start taking opioids.
N-001 numbs sensation. It lasts for up to 7 days, much longer than any anesthetic. “Our goal is to prolong the time before patients have to start opioids,” Blum says. “The hope is that they can switch from an anesthetic to our drug and thereby decrease the likelihood they're going to take the opioid in the first place.”
Their latest animal trial showed promising results. In mice, N-001 reduced pain-like behaviour by 90 percent compared to the control group. One dose became effective in two hours and lasted a week. A high dose had pain-relieving effects similar to an opioid.
Professor Stephen P. Cohen, director of pain operations at John Hopkins, believes the Neurocarrus approach has potential but highlights the need to go beyond animal testing. “While I think it's promising, it's an uphill battle,” he says. “They have shown some efficacy comparable to opioids, but animal studies don't translate well to people.”
Nadeau, the University of Florida neurologist, agrees. “It will be a long path to get to a successful clinical trial in humans. But it presents a very novel approach to pain reduction.”
Blum is now awaiting approval for phase I clinical trials for acute pain. He also hopes to start testing the drug's effect on chronic pain.
Learning from people who feel no pain
Like Blum, a pharmaceutical company called Vertex is focusing on treating acute pain after surgery. But they’re doing this in a different way, by targeting a sodium channel that plays a critical role in transmitting pain signals.
In 2004, Stephen Waxman, a neurology professor at Yale, led a search for genetic pain anomalies and found that biologically related people who felt no pain despite fractures, burns and even childbirth had mutations in the Nav1.7 sodium channel. Further studies in other families who experienced no pain showed similar mutations in the Nav1.8 sodium channel.
Scientists set out to modify these channels. Many unsuccessful efforts followed, but Vertex has now developed VX-548, a medicine to inhibit Nav1.8. Typically, sodium ions flow through sodium channels to generate rapid changes in voltage which create electrical pulses. When pain is detected, these pulses in the Nav1.8 channel transmit pain signals. VX-548 uses small molecules to inhibit the channel from opening. This blocks the flow of sodium ions and the pain signal. Because Nav1.8 operates only in peripheral nerves, located outside the brain, VX-548 can relieve pain without any risk of addiction.
"Frankly we need drugs for chronic pain more than acute pain," says Waxman.
The team just finished phase II clinical trials for patients following abdominoplasty surgery and bunionectomy surgery.
After abdominoplasty surgery, 76 patients were treated with a high dose of VX-548. Researchers then measured its effectiveness in reducing pain over 48 hours, using the SPID48 scale, in which higher scores are desirable. The score for Vertex’s drug was 110.5 compared to 72.7 in the placebo group, whereas the score for patients taking an opioid was 85.2. The study involving bunionectomy surgery showed positive results as well.
Waxman, who has been at the forefront of studies into Nav1.7 and Nav1.8, believes that Vertex's results are promising, though he highlights the need for further clinical trials.
“Blocking Nav1.8 is an attractive target,” he says. “[Vertex is] studying pain that is relatively simple and uniform, and that's key to having a drug trial that is informative. But the study needs to be replicated and frankly we need drugs for chronic pain more than acute pain. If this is borne out by additional studies, it's one important step in a journey.”
Vertex will be launching phase III trials later this year.
Finding just the right amount of Nerve Growth Factor
Whereas Neurocarrus and Vertex are targeting short-term pain, a company called Levicept is concentrating on relieving chronic osteoarthritis pain. Around 32.5 million Americans suffer from osteoarthritis. Patients commonly take NSAIDs, or non-steroidal anti-inflammatory drugs, but they cannot be taken long-term. Some take opioids but they aren't very effective.
Levicept’s drug, Levi-04, is designed to modify a signaling pathway associated with pain. Nerve Growth Factor (NGF) is a neurotrophin: it’s involved in nerve growth and function. NGF signals by attaching to receptors. In pain there are excess neurotrophins attaching to receptors and activating pain signals.
“What Levi-04 does is it returns the natural equilibrium of neurotrophins,” says Simon Westbrook, the CEO and founder of Levicept. It stabilizes excess neurotrophins so that the NGF pathway does not signal pain. Levi-04 isn't addictive since it works within joints and in nerves outside the brain.
Westbrook was initially involved in creating an anti-NGF molecule for Pfizer called Tanezumab. At first, Tanezumab seemed effective in clinical trials and other companies even started developing their own versions. However, a problem emerged. Tanezumab caused rapidly progressive osteoarthritis, or RPOA, in some patients because it completely removed NGF from the system. NGF is not just involved in pain signalling, it’s also involved in bone growth and maintenance.
Levicept has found a way to modify the NGF pathway without completely removing NGF. They have now finished a small-scale phase I trial mainly designed to test safety rather than efficacy. “We demonstrated that Levi-04 is safe and that it bound to its target, NGF,” says Westbrook. It has not caused RPOA.
Professor Philip Conaghan, director of the Leeds Institute of Rheumatic and Musculoskeletal Medicine, believes that Levi-04 has potential but urges the need for caution. “At this early stage of development, their molecule looks promising for osteoarthritis pain,” he says. “They will have to watch out for RPOA which is a potential problem.”
Westbrook starts phase II trials with 500 patients this summer to check for potential side effects and test the drug’s efficacy.
There is a real push to find an effective alternative to opioids. “We have a lot of work to do,” says Professor Waxman. “But I am confident that we will be able to develop new, much more effective pain therapies.”
Tech-related injuries are becoming more common as many people depend on - and often develop addictions for - smart phones and computers.
In the 1990s, a mysterious virus spread throughout the Massachusetts Institute of Technology Artificial Intelligence Lab—or that’s what the scientists who worked there thought. More of them rubbed their aching forearms and massaged their cricked necks as new computers were introduced to the AI Lab on a floor-by-floor basis. They realized their musculoskeletal issues coincided with the arrival of these new computers—some of which were mounted high up on lab benches in awkward positions—and the hours spent typing on them.
Today, these injuries have become more common in a society awash with smart devices, sleek computers, and other gadgets. And we don’t just get hurt from typing on desktop computers; we’re massaging our sore wrists from hours of texting and Facetiming on phones, especially as they get bigger in size.
In 2007, the first iPhone measured 3.5-inches diagonally, a measurement known as the display size. That’s been nearly doubled by the newest iPhone 13 Pro, which has a 6.7-inch display. Other phones, too, like the Google Pixel 6 and the Samsung Galaxy S22, have bigger screens than their predecessors. Physical therapists and orthopedic surgeons have had to come up with names for a variety of new conditions: selfie elbow, tech neck, texting thumb. Orthopedic surgeon Sonya Sloan says she sees selfie elbow in younger kids and in women more often than men. She hears complaints related to technology once or twice a day.
The addictive quality of smartphones and social media means that people spend more time on their devices, which exacerbates injuries. According to Statista, 68 percent of those surveyed spent over three hours a day on their phone, and almost half spent five to six hours a day. Another report showed that people dedicate a third of their day to checking their phones, while the Media Effects Research Laboratory at Pennsylvania State University has found that bigger screens, ideal for entertainment purposes, immerse their users more than smaller screens. Oversized screens also provide easier navigation and more space for those with bigger hands or trouble seeing.
But others with conditions like arthritis can benefit from smaller phones. In March of 2016, Apple released the iPhone SE with a display size of 4.7 inches—an inch smaller than the iPhone 7, released that September. Apple has since come out with two more versions of the diminutive iPhone SE, one in 2020 and another in 2022.
These devices are now an inextricable part of our lives. So where does the burden of responsibility lie? Is it with consumers to adjust body positioning, get ergonomic workstations, and change habits to abate tech-related pain? Or should tech companies be held accountable?
Kavin Senapathy, a freelance science journalist, has the Google Pixel 6. She was drawn to the phone because Google marketed the Pixel 6’s camera as better at capturing different skin tones. But this phone boasts one of the largest display sizes on the market: 6.4 inches.
Senapathy was diagnosed with carpal and cubital tunnel syndromes in 2017 and fibromyalgia in 2019. She has had to create a curated ergonomic workplace setup, otherwise her wrists and hands get weak and tingly, and she’s had to adjust how she holds her phone to prevent pain flares.
Recently, Senapathy underwent an electromyography, or an EMG, in which doctors insert electrodes into muscles to measure their electrical activity. The electrical response of the muscles tells doctors whether the nerve cells and muscles are successfully communicating. Depending on her results, steroid shots and even surgery might be required. Senapathy wants to stick with her Pixel 6, but the pain she’s experiencing may push her to buy a smaller phone. Unfortunately, options for these modestly sized phones are more limited.
These devices are now an inextricable part of our lives. So where does the burden of responsibility lie? Is it with consumers like Senapathy to adjust body positioning, get ergonomic workstations, and change habits to abate tech-related pain? Or should tech companies be held accountable for creating addictive devices that lead to musculoskeletal injury?

Kavin Senapathy, a freelance journalist, bought the Google Pixel 6 because of its high-quality camera, but she’s had to adjust how she holds the oversized phone to prevent pain flares.
Kavin Senapathy
A one-size-fits-all mentality for smartphones will continue to lead to injuries because every user has different wants and needs. S. Shyam Sundar, the founder of Penn State’s lab on media effects and a communications professor, says the needs for mobility and portability conflict with the desire for greater visibility. “The best thing a company can do is offer different sizes,” he says.
Joanna Bryson, an AI ethics expert and professor at The Hertie School of Governance in Berlin, Germany, echoed these sentiments. “A lot of the lack of choice we see comes from the fact that the markets have consolidated so much,” she says. “We want to make sure there’s sufficient diversity [of products].”
Consumers can still maintain some control despite the ubiquity of tech. Sloan, the orthopedic surgeon, has to pester her son to change his body positioning when using his tablet. Our heads get heavier as they bend forward: at rest, they weigh 12 pounds, but bent 60 degrees, they weigh 60. “I have to tell him, ‘Raise your head, son!’” she says. It’s important, Sloan explains, to consider that growth and development will affect ligaments and bones in the neck, potentially making kids even more vulnerable to injuries from misusing gadgets. She recommends that parents limit their kids’ tech time to alleviate strain. She also suggested that tech companies implement a timer to remind us to change our body positioning.
In 2017, Nan-Wei Gong, a former contractor for Google, founded Figur8, which uses wearable trackers to measure muscle function and joint movement. It’s like physical therapy with biofeedback. “Each unique injury has a different biomarker,” says Gong. “With Figur8, you are comparing yourself to yourself.” This allows an individual to self-monitor for wear and tear and strengthen an injury in a way that’s efficient and designed for their body. Gong noticed that the work-from-home model during the COVID-19 pandemic created a new set of ergonomic problems that resulted in injuries. Figur8 provides real-time data for these injuries because “behavioral change requires feedback.”
Gong worked on a project called Jacquard while at Google. Textile experts weave conductive thread into their fabric, and the result is a patch of the fabric—like the cuff of a Levi’s jacket—that responds to commands on your smartphone. One swipe can call your partner or check the weather. It was designed with cyclists in mind who can’t easily check their phones, and it’s part of a growing movement in the tech industry to deliver creative, hands-free design. Gong thinks that engineers at large corporations like Google have accessibility in mind; it’s part of what drives their decisions for new products.
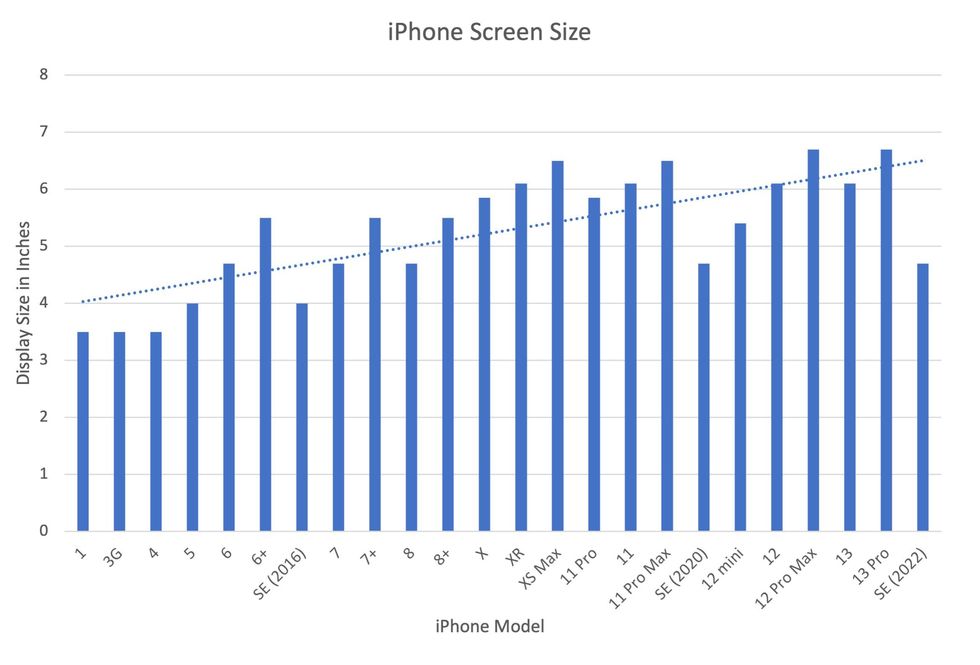
Display sizes of iPhones have become larger over time.
Sourced from Screenrant https://screenrant.com/iphone-apple-release-chronological-order-smartphone/ and Apple Tech Specs: https://www.apple.com/iphone-se/specs/
Back in Germany, Joanna Bryson reminds us that products like smartphones should adhere to best practices. These rules may be especially important for phones and other products with AI that are addictive. Disclosure, accountability, and regulation are important for AI, she says. “The correct balance will keep changing. But we have responsibilities and obligations to each other.” She was on an AI Ethics Council at Google, but the committee was disbanded after only one week due to issues with one of their members.
Bryson was upset about the Council’s dissolution but has faith that other regulatory bodies will prevail. OECD.AI, and international nonprofit, has drafted policies to regulate AI, which countries can sign and implement. “As of July 2021, 46 governments have adhered to the AI principles,” their website reads.
Sundar, the media effects professor, also directs Penn State’s Center for Socially Responsible AI. He says that inclusivity is a crucial aspect of social responsibility and how devices using AI are designed. “We have to go beyond first designing technologies and then making them accessible,” he says. “Instead, we should be considering the issues potentially faced by all different kinds of users before even designing them.”