Trading syphilis for malaria: How doctors treated one deadly disease by infecting patients with another

In the 1920s, doctors induced a high fever in patients - so called "fever therapy" - as a way to help them recover from syphilis, though it involved ethical problems.
If you had lived one hundred years ago, syphilis – a bacterial infection spread by sexual contact – would likely have been one of your worst nightmares. Even though syphilis still exists, it can now be detected early and cured quickly with a course of antibiotics. Back then, however, before antibiotics and without an easy way to detect the disease, syphilis was very often a death sentence.
To understand how feared syphilis once was, it’s important to understand exactly what it does if it’s allowed to progress: the infections start off as small, painless sores or even a single sore near the vagina, penis, anus, or mouth. The sores disappear around three to six weeks after the initial infection – but untreated, syphilis moves into a secondary stage, often presenting as a mild rash in various areas of the body (such as the palms of a person’s hands) or through other minor symptoms. The disease progresses from there, often quietly and without noticeable symptoms, sometimes for decades before it reaches its final stages, where it can cause blindness, organ damage, and even dementia. Research indicates, in fact, that as much as 10 percent of psychiatric admissions in the early 20th century were due to dementia caused by syphilis, also known as neurosyphilis.
Like any bacterial disease, syphilis can affect kids, too. Though it’s spread primarily through sexual contact, it can also be transmitted from mother to child during birth, causing lifelong disability.
The poet-physician Aldabert Bettman, who wrote fictionalized poems based on his experiences as a doctor in the 1930s, described the effect syphilis could have on an infant in his poem Daniel Healy:
I always got away clean
when I went out
With the boys.
The night before
I was married
I went out,—But was not so fortunate;
And I infected
My bride.
When little Daniel
Was born
His eyes discharged;
And I dared not tell
That because
I had seen too much
Little Daniel sees not at all
Given the horrors of untreated syphilis, it’s maybe not surprising that people would go to extremes to try and treat it. One of the earliest remedies for syphilis, dating back to 15th century Naples, was using mercury – either rubbing it on the skin where blisters appeared, or breathing it in as a vapor. (Not surprisingly, many people who underwent this type of “treatment” died of mercury poisoning.)
Other primitive treatments included using tinctures made of a flowering plant called guaiacum, as well as inducing “sweat baths” to eliminate the syphilitic toxins. In 1910, an arsenic-based drug called Salvarsan hit the market and was hailed as a “magic bullet” for its ability to target and destroy the syphilis-causing bacteria without harming the patient. However, while Salvarsan was effective in treating early-stage syphilis, it was largely ineffective by the time the infection progressed beyond the second stage. Tens of thousands of people each year continued to die of syphilis or were otherwise shipped off to psychiatric wards due to neurosyphilis.
It was in one of these psychiatric units in the early 20th century that Dr. Julius Wagner-Juaregg got the idea for a potential cure.
Wagner-Juaregg was an Austrian-born physician trained in “experimental pathology” at the University of Vienna. Wagner-Juaregg started his medical career conducting lab experiments on animals and then moved on to work at different psychiatric clinics in Vienna, despite having no training in psychiatry or neurology.
Wagner-Juaregg’s work was controversial to say the least. At the time, medicine – particularly psychiatric medicine – did not have anywhere near the same rigorous ethical standards that doctors, researchers, and other scientists are bound to today. Wagner-Juaregg would devise wild theories about the cause of their psychiatric ailments and then perform experimental procedures in an attempt to cure them. (As just one example, Wagner-Juaregg would sterilize his adolescent male patients, thinking “excessive masturbation” was the cause of their schizophrenia.)
But sometimes these wild theories paid off. In 1883, during his residency, Wagner-Juaregg noted that a female patient with mental illness who had contracted a skin infection and suffered a high fever experienced a sudden (and seemingly miraculous) remission from her psychosis symptoms after the fever had cleared. Wagner-Juaregg theorized that inducing a high fever in his patients with neurosyphilis could help them recover as well.
Eventually, Wagner-Juaregg was able to put his theory to the test. Around 1890, Wagner-Juaregg got his hands on something called tuberculin, a therapeutic treatment created by the German microbiologist Robert Koch in order to cure tuberculosis. Tuberculin would later turn out to be completely ineffective for treating tuberculosis, often creating severe immune responses in patients – but for a short time, Wagner-Juaregg had some success in using tuberculin to help his dementia patients. Giving his patients tuberculin resulted in a high fever – and after completing the treatment, Wagner-Jauregg reported that his patient’s dementia was completely halted. The success was short-lived, however: Wagner-Juaregg eventually had to discontinue tuberculin as a treatment, as it began to be considered too toxic.
By 1917, Wagner-Juaregg’s theory about syphilis and fevers was becoming more credible – and one day a new opportunity presented itself when a wounded soldier, stricken with malaria and a related fever, was accidentally admitted to his psychiatric unit.
When his findings were published in 1918, Wagner-Juaregg’s so-called “fever therapy” swept the globe.
What Wagner-Juaregg did next was ethically deplorable by any standard: Before he allowed the soldier any quinine (the standard treatment for malaria at the time), Wagner-Juaregg took a small sample of the soldier’s blood and inoculated three syphilis patients with the sample, rubbing the blood on their open syphilitic blisters.
It’s unclear how well the malaria treatment worked for those three specific patients – but Wagner-Juaregg’s records show that in the span of one year, he inoculated a total of nine patients with malaria, for the sole purpose of inducing fevers, and six of them made a full recovery. Wagner-Juaregg’s treatment was so successful, in fact, that one of his inoculated patients, an actor who was unable to work due to his dementia, was eventually able to find work again and return to the stage. Two additional patients – a military officer and a clerk – recovered from their once-terminal illnesses and returned to their former careers as well.
When his findings were published in 1918, Wagner-Juaregg’s so-called “fever therapy” swept the globe. The treatment was hailed as a breakthrough – but it still had risks. Malaria itself had a mortality rate of about 15 percent at the time. Many people considered that to be a gamble worth taking, compared to dying a painful, protracted death from syphilis.
Malaria could also be effectively treated much of the time with quinine, whereas other fever-causing illnesses were not so easily treated. Triggering a fever by way of malaria specifically, therefore, became the standard of care.
Tens of thousands of people with syphilitic dementia would go on to be treated with fever therapy until the early 1940s, when a combination of Salvarsan and penicillin caused syphilis infections to decline. Eventually, neurosyphilis became rare, and then nearly unheard of.
Despite his contributions to medicine, it’s important to note that Wagner-Juaregg was most definitely not a person to idolize. In fact, he was an outspoken anti-Semite and proponent of eugenics, arguing that Jews were more prone to mental illness and that people who were mentally ill should be forcibly sterilized. (Wagner-Juaregg later became a Nazi sympathizer during Hitler’s rise to power even though, bizarrely, his first wife was Jewish.) Another problematic issue was that his fever therapy involved experimental treatments on many who, due to their cognitive issues, could not give informed consent.
Lack of consent was also a fundamental problem with the syphilis study at Tuskegee, appalling research that began just 14 years after Wagner-Juaregg published his “fever therapy” findings.
Still, despite his outrageous views, Wagner-Juaregg was awarded the Nobel Prize in Medicine or Physiology in 1927 – and despite some egregious human rights abuses, the miraculous “fever therapy” was partly responsible for taming one of the deadliest plagues in human history.
George Papanicolaou (1883-1962), Greek-born American physician developed a simple cytological test for cervical cancer in 1928.
For decades, women around the world have made the annual pilgrimage to their doctor for the dreaded but potentially life-saving Papanicolaou test, a gynecological exam to screen for cervical cancer named for Georgios Papanicolaou, the Greek immigrant who developed it.
The Pap smear, as it is commonly known, is credited for reducing cervical cancer mortality by 70% since the 1960s; the American Cancer Society (ACS) still ranks the Pap as the most successful screening test for preventing serious malignancies. Nonetheless, the agency, as well as other medical panels, including the US Preventive Services Task Force and the American College of Obstetrics and Gynecology are making a strong push to replace the Pap with the more sensitive high-risk HPV screening test for the human papillomavirus virus, which causes nearly all cases of cervical cancer.
So, how was the Pap developed and how did it become the gold standard of cervical cancer detection for more than 60 years?
Born on May 13, 1883, on the island of Euboea, Greece, Georgios Papanicolaou attended the University of Athens where he majored in music and the humanities before earning his medical degree in 1904 and PhD from the University of Munich six years later. In Europe, Papanicolaou was an assistant military surgeon during the Balkan War, a psychologist for an expedition of the Oceanographic Institute of Monaco and a caregiver for leprosy patients.
When he and his wife, Andromache Mavroyenous (Mary), arrived at Ellis Island on October 19, 1913, the young couple had scarcely more than the $250 minimum required to immigrate, spoke no English and had no job prospects. They worked a series of menial jobs--department store sales clerk, rug salesman, newspaper clerk, restaurant violinist--before Papanicolaou landed a position as an anatomy assistant at Cornell University and Mary was hired as his lab assistant, an arrangement that would last for the next 50 years.
Papanikolaou would later say the discovery "was one of the greatest thrills I ever experienced during my scientific career."
In his early research, Papanikolaou used guinea pigs to prove that gender is determined by the X and Y chromosomes. Using a pediatric nasal speculum, he collected and microscopically examined vaginal secretions of guinea pigs, which revealed distinct cell changes connected to the menstrual cycle. He moved on to study reproductive patterns in humans, beginning with his faithful wife, Mary, who not only endured his almost-daily cervical exams for decades, but also recruited friends as early research participants.
Writing in the medical journal Growth in 1920, the scientist outlined his theory that a microscopic smear of vaginal fluid could detect the presence of cancer cells in the uterus. Papanikolaou would later say the discovery "was one of the greatest thrills I ever experienced during my scientific career."
At this time, cervical cancer was the number one cancer killer of American women but physicians were skeptical of these new findings. They continued to rely on biopsy and curettage to diagnose and treat the disease until Papanicolaou's discovery was published in American Journal of Obstetrics and Gynecology. An inexpensive, easy-to-perform test that could detect cervical cancer, precancerous dysplasia and other cytological diseases was a sea change. Between 1975 and 2001, the cervical cancer rate was cut in half.
Papanicolaou became Emeritus Professor at Cornell University Medical College and received numerous awards, including the Albert Lasker Award for Clinical Medical Research and the Medal of Honor from the American Cancer Society. His image was featured on the Greek currency and the US Post Office issued a commemorative stamp in his honor. But international acclaim didn't lead to a more relaxed schedule. The researcher continued to work seven days a week and refused to take vacations.
After nearly 50 years, Papanicolaou left Cornell to head and develop the Cancer Institute of Miami. He died of a heart attack on February 19, 1962, just three months after his arrival. Mary continued to work in the renamed Papanicolaou Cancer Research Institute until her death 20 years later.
The annual pap smear was originally tied to renewing a birth control prescription. Canada began recommending Pap exams every three years in 1978. The United States followed suit in 2012, noting that it takes many years for cervical cancer to develop. In September 2020, the American Cancer Society recommended delaying the first gynecological pelvic exam until age 25 and replacing the Pap test completely with the more accurate human papillomavirus (HPV) test every five years as the technology becomes more widely available.
Not everyone agrees that it's time to do away with this proven screening method, though. The incidence rate of cervical cancer among Hispanic women is 28% higher than for white women, and Black women are more likely to die of cervical cancer than any other racial or ethnicities.
Whether the Pap is administered every year, every three years or not at all, Papanicolaou will always be known as the medical hero who saved countless women who would otherwise have succumbed to cervical cancer.
Science Has Given Us the Power to Undermine Nature's Deadliest Creature: Should We Use It?
The Aedes aegypti mosquito, which can carry devastating diseases, was recently engineered by a biotech company to have a genetic "kill switch" intended to crash the local population in the Florida Keys.
Lurking among the swaying palm trees, sugary sands and azure waters of the Florida Keys is the most dangerous animal on earth: the mosquito.
While there are thousands of varieties of mosquitoes, only a small percentage of them are responsible for causing disease. One of the leading culprits is Aedes aegypti, which thrives in the warm standing waters of South Florida, Central America and other tropical climes, and carries the viruses that cause yellow fever, dengue, chikungunya and Zika.
Dengue, a leading cause of death in many Asian and Latin American countries, causes bleeding and pain so severe that it's referred to as "breakbone fever." Chikungunya and yellow fever can both be fatal, and Zika, when contracted by a pregnant woman, can infect her fetus and cause devastating birth defects, including a condition called microcephaly. Babies born with this condition have abnormally small heads and lack proper brain development, which leads to profound, lifelong disabilities.
Decades of efforts to eradicate the disease-carrying Aedes aegypti mosquito from the Keys and other tropical locales have had limited impact. Since the advent of pesticides, homes and neighborhoods have been drenched with them, but after each spraying, the mosquito population quickly bounces back, and the pesticides have to be sprayed over and over. But thanks to genetic engineering, new approaches are underway that could possibly prove safer, cheaper and more effective than any pesticide.
One of those approaches involves, ironically, releasing more mosquitoes in the Florida Keys.
The kill-switch will ensure that the female offspring die before they reach maturity and thus, be unable to reproduce.
British biotech company Oxitec has engineered male mosquitoes to have a genetic "kill-switch" that could potentially crash the local population of Aedes aegypti, at least in the short-term. The modified males that are being released are intended to mate with wild females.
Males don't bite; it's the female that's deadly, always seeking out blood to gorge on to help mature her eggs. After settling her filament-thin legs on her prey, she sinks a needlelike proboscis into the skin and sucks the blood until her translucent belly is bloated and glowing red.
The kill-switch will ensure that the female offspring die before they reach maturity and thus, be unable to reproduce. In some experiments using genetically modified mosquitoes, the small number of females that survived were rendered unable to bite. The modification prevented the proboscis, the sickle-like needle that pierces the skin, from forming properly. But this isn't the case with Oxitec's mosquitoes; in the Oxitec release, the females simply die off before they can mate.
The modified mosquitoes are the second genetically engineered insect to be released in the U.S. by Oxitec. The first was a modified diamondback moth, an agricultural pest that doesn't bite humans. But with the mosquitoes, there are many questions about the long-term effects on wild ecosystems, other species in the food chain, and human health. With the Keys initiative, there has been vociferous opposition from environmental groups and some local residents, but some scientists and public health experts say that genetically modified insects pose less of a risk than the diseases they carry and the powerful, indiscriminant pesticides used to combat them.
Oxitec spent a decade developing the technology and engaging in a massive public education campaign before beginning the field test in April. Eventually, the company will release 750,000 of the insects from six locations on three islands of the Florida Keys. Although the release has been approved by the Environmental Protection Agency, the Florida Department of Agriculture and Consumer Services, and the Florida Keys Mosquito Control District, the company was never able to obtain unanimous approval among local residents, some of whom worry that the experiment could cause irreversible damage to the ecosystem.
The company has already begun distributing multiple blue and white boxes containing the eggs of thousands of the mosquitoes which, when water is added, will hatch legions of modified males.
There are a number of techniques available to genetically engineer animals and plants to minimize disease and maximize crop yields. According to Kevin Gorman, chief development officer for Oxitec, the company's mosquitoes were altered by injecting genetic material into the eggs, testing them, then re-injecting them if not enough of the new genes were incorporated into the developing embryos. "We insert genes, but take nothing away," he says.
Gorman points out that the Oxitec mosquitoes will only pass the kill-switch genes on to some of their offspring, and that they will die out fairly quickly. They should temporarily lessen diseases by reducing the local population of Aedes aegypti, but to have a long-term effect, repeated introductions of the altered mosquitoes would have to take place.
Critics say the Oxitec experiment is a precursor to a far more consequential, and more troubling development: the introduction of gene drives in modified species that aggressively tilt inheritance factors in a decided direction.
Gene Drives
Gene drives coupled with the recent development of the gene-editing technique, CRISPR-Cas9, promise to be far more targeted and powerful than previous gene altering efforts. Gene drives override the normal laws of inheritance by harnessing natural processes involved in reproduction. The technique targets small sections of the animal's DNA and replaces it with an altered allele, or trait-determining snippet. Normally, when two members of a species mate, the offspring have a 50 percent chance of receiving an allele because they will receive one from each parent. But in a gene drive, each offspring ends up getting two copies of a desired allele from a single parent—the modified parent. The method "drives" the modified DNA into up to 100 percent of the animals' offspring.
In the case of gene drive mosquitoes, the modified males will mate with wild females. Upon fertilization of the egg, the offspring will start off with one copy of the targeted allele from each parent. But an enzyme, called Cas9, is introduced and acts as a kind of molecular scissors to cut, or damage, the "wild" allele. Then the developing embryo's genetic repair mechanisms kick in and, to repair the damage, copy the undamaged allele from the modified parent. In this way, the offspring ends up with two copies of the modified allele, and it will pass the modification on to virtually all of its progeny.
There is some debate among researchers and others about what constitutes a gene drive, but leaders in the nascent field, such as Andrea Crisanti, generally agree that the defining factor is the heritability of a change introduced into a species. A gene drive is not a particular gene or suite of genes, but a program that proliferates in a species because it is inherited by virtually all offspring.
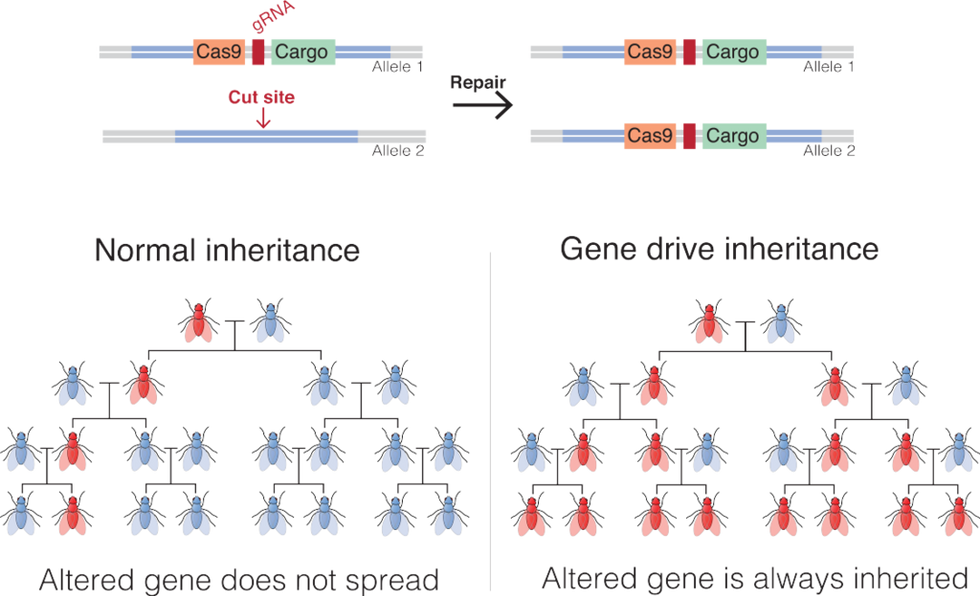
An illustration of how gene drives spread an altered gene through a population.
Mariuswalter, CC BY-SA 4.0 <https://creativecommons.org/licenses/by-sa/4.0>, via Wikimedia Commons
Of the experts who spoke with Leaps.org for this article, there was disagreement on whether the Oxitec mosquitoes carry a gene drive, but Gorman says they don't because they carry no inheritance advantage. The mosquitoes have baked-in limitations on their potential impact on the tropical ecosystem because the kill-switch should only temporarily affect the local population of Aedes aegypti. The modified mosquitoes will die pretty quickly. But modified organisms that do carry gene drives have the potential to spread widely and persist for an unknown period of time.
Since it has such a reproductive advantage, animals modified by CRISPR and carrying gene drives can quickly replace wild species that compete with them. On the other hand, if the gene drive carries a kill-switch, it can theoretically cause a whole species to collapse.
This makes many people uneasy in an age of mass extinctions, when animals and ecosystems are already under extreme stress due to climate change and the ceaseless destruction of their habitats. Ecosystems are intricate, delicately balanced mosaics where one animal's competitor is another animal's food. The interconnectedness of nature is only partially understood and still contains many mysteries as to what effects human intervention could eventually cause.
But there's a compelling case to be made for the use of gene drives in general. Economies throughout the world are often based on the ecosystem and its animals, which rely on a natural food chain that was evolved over billions of years. But diseases carried by mosquitoes and other animals cause massive damage, both economically and in terms of human suffering.
Malaria alone is a case in point. In 2019, the World Health Organization reported 229 million cases of malaria, which led to 449,000 deaths worldwide. Over 70 percent of those deaths were in children under the age of 12. Efforts to combat malaria-carrying mosquitoes rely on fogging the home with chemical pesticides and sleeping under pesticide-soaked nets, and while this has reduced the occurrence of malaria in recent years, the result is nowhere near as effective as eradicating the Anopheles gambiae mosquito that carries the disease.
Pesticides, a known carcinogen for animals and humans, are a blunt instrument, says Anthony Shelton, a biologist and entomologist at Cornell University. "There are no pesticides so specific that they just get the animal you want to target. They get pollinators. They get predators and parasites. They negatively affect the ecosystem, and they get into our bodies." And it's not uncommon for insects to develop resistance to pesticides, necessitating the continuous development of new, more powerful chemicals to control them.
"The harm of insecticides is not debatable," says Shelton. With gene drives, the potential harm is less clear.
Shelton also points out that although genetic modification sounds radical, people have been altering the genes of animals since before recorded history, through the selective breeding of farm and domesticated animals. While critics of genetic modification decry the possibility of changing the trajectory of evolution in animals, "We've been doing it for centuries," says Shelton. "Gene drives are just a much faster way to do what we've been doing all along."
Still, one might argue that farms are closed experiments, because animals enclosed within farms don't mate with wild animals. This limits the impact of human changes on the larger ecosystem. And getting new genes to work their way through multiple generations in longer-lived animals through breeding can take centuries, which imposes the element of time to ascertain the relative benefits of any introduced change. Gene drives fast-forward change in ways that have never been harnessed before.
The unique thing about gene drives, Shelton says, is that they only affect the targeted species, because those animals will only breed with their own species. Although the Oxitec mosquitoes are modified but not imbued with a gene drive, they illustrate the point. Aedes aegypti will only mate with its own species, and not with any of the other 3,000 varieties of mosquito. According to Shelton, "If they were to disappear, it would have no effect on the fish, bats and birds that feed on them." But should gene drives become widely used, this won't always be true of animals that play a larger part in the food chain. This will be especially true if gene drives are used in mammals.
One factor, cited by both proponents of gene drives and those who want a complete moratorium on them, is that once a gene drive is released into the wild, animals tend to evolve strategies to resist them. In a 2017 article in Nature, Philip Messer, a population geneticist at Cornell, says that gene drives create "the ideal conditions for resistant organisms to flourish."
Sometimes, when CRISPR is used and the Cas9 enzyme cuts an allele soon after egg fertilization, the animal's repair mechanism, rather than creating a straight copy of the desired allele, inserts random DNA letters. The gene drive won't recognize the new sequence, and the change will slip through. In this way, nature has a way of overriding gene drives.
In caged experiments using CRISPR-modified mosquitoes, while the gene drive initially worked, resistance has developed fairly rapidly. Scientists working for Target Malaria, the massive anti-malaria enterprise funded by the Bill and Melinda Gates Foundation, are now working on developing a new version of a gene drive that is not so vulnerable to genetic resistance. But cage conditions are not representative of complex natural ecosystems, and to figure out how a modified species is going to affect the big picture, ultimately they will have to be tested in the wild.
Because there are so many unknowns, such testing is just too dangerous to undertake, according to environmentalists such as Dana Perls of the Friends of the Earth, an international consortium of environmental organizations headquartered in Amsterdam. "There's no safe way to experiment in the wild," she says. "Extinction is permanent, and to drive any species to extinction could have major environmental problems. At a time when we're seeing species disappearing at a high rate, we need to focus on safe processes and a slow approach rather than assume there's a silver bullet."
She cites a number of possible harmful outcomes from genetic modification, including the possible creation of dangerous hybrids that could be more effective at spreading disease and more resistant to pesticides. She points to a 2019 paper in Scientific Reports in which Yale researchers suggested there's evidence that genetically modified species can interbreed with organisms outside their own species. The researchers claimed that when Oxitec tested its modified Aedes aegypti mosquitoes in Brazil, the release resulted in a dangerous hybrid due to the altered animals breeding with two other varieties of mosquito. They suggested that the hybrid mosquito was more robust than the original gene drive mosquitoes.
The paper contributed to breathless headlines in the media and made a big splash with the anti-GMO community. However, it turned out that when other scientists reviewed the data, they found it didn't support the authors' claims. In a short time, the editors of Nature ran an Editorial Expression of Concern for the article, noting that of the insects examined by the researchers, none of them contained the transgenes of the released mosquitoes. Among multiple concerns, Nature found that the researchers didn't follow the released population for more than a short time, and that previous work from the same authors had shown that after a short time, transgenes would have faded from the population.
Of course, unintended consequences are always a concern any time we interfere with nature, says Michael Montague, a senior scholar at Johns Hopkins University's Center for Health Security. "Unpredictability is part of living in the world," he says. Still, he's relatively comfortable with the limited Florida Keys release.
"Even if one type of mosquito was eliminated in the Keys, the ecosystem wouldn't notice," he says. This is because of the thousands of other species of mosquito. He says that while the Keys initiative is ultimately a test, "Oxitec has done their due diligence."
Montague addressed another concern voiced by Perls. The Oxitec mosquitoes were developed so that the female larvae will only hatch in water containing the antibiotic tetracycline. Perls and others caution that, because of the widespread use of antibiotics, the drug inevitably makes its way into the water system, and could be present in the standing pools of water that mosquitoes mate and lay their eggs in.
It's highly unlikely that tetracycline would exist in concentrations high enough to make any difference, says Montague. "But even if it did happen, and the modified females hatched out and mated with wild males, many of their offspring would inherit the modification and only be able to hatch in tetracycline-laced water. The worst-case scenario would be that the pest control didn't work. Net effect: Zero," he says.
As for comparing GMO mosquitoes with insecticides, Montague says, "We 100 percent know insecticides have a harmful effect on human health, whereas modified [male] mosquitoes don't bite humans. They're essentially a chemical-free insecticide, and if there were to be some harmful effect on human health, it would have to be some complicated, convoluted effect" that no one has predicted.
It's not clear, though, given the transitory nature of self-limiting genetically modified insects, whether any effects on the ecosystem would be long-lasting. Certainly in the case of the Oxitec mosquitoes, any effect on the environment would likely be subtle. However, there are other species that are far more important to the food chain, and humans have been greatly impacting them for centuries, sometimes with disastrous effects.
The world's oceans are particularly vulnerable to the effects of human actions. "Codfish used to dominate the North Atlantic ecosystem," says Montague, but due to overfishing, there were huge changes to that ecosystem, including the expansion of their prey—lobsters, crabs and shrimp. The whole system got out of balance." The fish illustrate the international nature of the issues related to gene drives, because wild species have few boundaries and a change in one region can easily spread far and wide.
On the other hand, gene drives can be used for beneficial purposes beyond eliminating disease-carrying species. They could also be used to combat invasive species, fight crop-destroying insects, promote biodiversity, and give a leg up to endangered species that would otherwise die out.
Today nearly 90 percent of the world's islands have been invaded by disease-carrying rodents that have over-multiplied and are driving other island species to extinction. Common rodents such as rats and mice normally encounter a large number of predators in mainland territories, and this controls their numbers. Once they are introduced into island ecosystems, however, they have few predators and often become invasive. Because of this, they are a prevalent cause of the extinction of both animals and plants globally. The primary way to combat them has been to spread powerful toxicants that, when ingested, cause death. Not only has this inhumane practice had limited impact, the toxicants can be eaten by untargeted species and are toxic to humans.
The Genetic Biocontrol of Invasive Rodents program (GBIRd), an international consortium of scientists, ethicists, regulatory experts, sociologists, conservationists and others, is exploring the possible development of a genetically modified mouse that could be introduced to islands where rodents are invasive. Similar to the Oxitec mosquitoes, the mice would carry a modification that results in the appearance of only one sex, and they would also carry a gene drive. Theoretically, once they mate with the wild mice, all of the surviving offspring would be either male or female, and the species would disappear from the islands, giving other, threatened species an opportunity to revive.
GBIRd is moving slowly by design and is currently focused on asking if a genetically engineered mouse should be developed. The program is a potential model for how gene drives can be ethically developed with maximum foresight and the least impact on complex ecosystems. By first releasing a genetically engineered mouse on an island — likely years from now — the impact would naturally be contained within a limited locale.
Regulating GM Insects
While multiple agencies in the U.S. were involved in approving the release of the Oxitec mosquitoes, most experts agree that there is not a straightforward path to regulating genetically modified organisms released into the environment. Clearly, international regulation is needed as genetically modified organisms are released into open environments like the air and the ocean.
The United Nations' Convention on Biological Diversity, which oversees environmental issues at an international level, recently met to continue a process of hammering out voluntary protocols concerning gene drives. Multiple nations have already signed on to already-established protocols, but the United States has not and, according to Montague, is not expected to. "The U.S. will never be signatory to CBD agreements because agricultural companies are huge businesses" that may not see them as in their best interests, he says. Bans or limitations on the release of genetically modified organisms could limit crop yields, for example, thereby limiting profits.
Even if every nation signed on to international regulations of gene drives, cooperation is voluntary. The regulations wouldn't prevent bad actors from using the technology in nefarious ways, such as developing gene drives that can be used as weapons, according to Perls. An example would be unleashing a genetically modified invasive insect to destroy the crops of enemy nations. Or the releasing of a swarm of disease-carrying insects. But in this scenario, it would be very hard to limit the genetically modified species to a specific environment, and the bad actors could be unleashing disaster on themselves.
Because of the risks of misuse, scientists disagree on whether to openly share their gene drive research with others. But Montague believes that there should be a universal registry of gene drives, because "one gene drive can mess up another one. Two groups using the same species should know about each other," he says.
Ultimately, the decision of whether and when to release gene drives into nature rests with not one group, but with society as a whole. This includes not only diverse experts and regulatory bodies, but the general public, a group Oxitec spent considerable time and resources interacting with for their Florida Keys project. In the end, they gained approval for the initiative by a majority of Keys residents, but never gained a total consensus.
There's no escaping the fact that the use of gene drives is a nascent field, and even geneticists and regulators are still grapping with the best ways to develop, oversee, regulate, and control them. Much more data is needed to fully ascertain its risks and benefits.
Experts agree that the Oxitec venture isn't likely to have a noticeable effect on the larger ecosystem unless something truly catastrophic goes wrong. But following the GMO mosquitoes over time will give scientists more real-world data about the long-term effects of genetically altered species. If the release doesn't work, nothing about the ecosystem will change and Aedes aegypti will continue to be a menace to human health. But if something goes horribly wrong, it could hinder the field for years, if not forever.
On the other hand, if the Oxitec mosquitoes and other early initiatives achieve their goals of reducing disease, increasing crop yields, and protecting biodiversity, in the words of Anthony Shelton, "Maybe, 25 to 50 years from now, people will wonder what all the fuss was about."
Correction: The original version of this article mistakenly stated that the modified Oxitec mosquitoes would not be able to form a proper proboscis to bite humans. That is true for some modified mosquitoes but not the Oxitec ones, whose female offspring die off before they reach maturity. Additionally, the Oxitec release was not approved by the FDA and CDC, as originally stated. The FDA and CDC withdrew their role and passed the oversight to other regulatory entities.

