Trading syphilis for malaria: How doctors treated one deadly disease by infecting patients with another

In the 1920s, doctors induced a high fever in patients - so called "fever therapy" - as a way to help them recover from syphilis, though it involved ethical problems.
If you had lived one hundred years ago, syphilis – a bacterial infection spread by sexual contact – would likely have been one of your worst nightmares. Even though syphilis still exists, it can now be detected early and cured quickly with a course of antibiotics. Back then, however, before antibiotics and without an easy way to detect the disease, syphilis was very often a death sentence.
To understand how feared syphilis once was, it’s important to understand exactly what it does if it’s allowed to progress: the infections start off as small, painless sores or even a single sore near the vagina, penis, anus, or mouth. The sores disappear around three to six weeks after the initial infection – but untreated, syphilis moves into a secondary stage, often presenting as a mild rash in various areas of the body (such as the palms of a person’s hands) or through other minor symptoms. The disease progresses from there, often quietly and without noticeable symptoms, sometimes for decades before it reaches its final stages, where it can cause blindness, organ damage, and even dementia. Research indicates, in fact, that as much as 10 percent of psychiatric admissions in the early 20th century were due to dementia caused by syphilis, also known as neurosyphilis.
Like any bacterial disease, syphilis can affect kids, too. Though it’s spread primarily through sexual contact, it can also be transmitted from mother to child during birth, causing lifelong disability.
The poet-physician Aldabert Bettman, who wrote fictionalized poems based on his experiences as a doctor in the 1930s, described the effect syphilis could have on an infant in his poem Daniel Healy:
I always got away clean
when I went out
With the boys.
The night before
I was married
I went out,—But was not so fortunate;
And I infected
My bride.
When little Daniel
Was born
His eyes discharged;
And I dared not tell
That because
I had seen too much
Little Daniel sees not at all
Given the horrors of untreated syphilis, it’s maybe not surprising that people would go to extremes to try and treat it. One of the earliest remedies for syphilis, dating back to 15th century Naples, was using mercury – either rubbing it on the skin where blisters appeared, or breathing it in as a vapor. (Not surprisingly, many people who underwent this type of “treatment” died of mercury poisoning.)
Other primitive treatments included using tinctures made of a flowering plant called guaiacum, as well as inducing “sweat baths” to eliminate the syphilitic toxins. In 1910, an arsenic-based drug called Salvarsan hit the market and was hailed as a “magic bullet” for its ability to target and destroy the syphilis-causing bacteria without harming the patient. However, while Salvarsan was effective in treating early-stage syphilis, it was largely ineffective by the time the infection progressed beyond the second stage. Tens of thousands of people each year continued to die of syphilis or were otherwise shipped off to psychiatric wards due to neurosyphilis.
It was in one of these psychiatric units in the early 20th century that Dr. Julius Wagner-Juaregg got the idea for a potential cure.
Wagner-Juaregg was an Austrian-born physician trained in “experimental pathology” at the University of Vienna. Wagner-Juaregg started his medical career conducting lab experiments on animals and then moved on to work at different psychiatric clinics in Vienna, despite having no training in psychiatry or neurology.
Wagner-Juaregg’s work was controversial to say the least. At the time, medicine – particularly psychiatric medicine – did not have anywhere near the same rigorous ethical standards that doctors, researchers, and other scientists are bound to today. Wagner-Juaregg would devise wild theories about the cause of their psychiatric ailments and then perform experimental procedures in an attempt to cure them. (As just one example, Wagner-Juaregg would sterilize his adolescent male patients, thinking “excessive masturbation” was the cause of their schizophrenia.)
But sometimes these wild theories paid off. In 1883, during his residency, Wagner-Juaregg noted that a female patient with mental illness who had contracted a skin infection and suffered a high fever experienced a sudden (and seemingly miraculous) remission from her psychosis symptoms after the fever had cleared. Wagner-Juaregg theorized that inducing a high fever in his patients with neurosyphilis could help them recover as well.
Eventually, Wagner-Juaregg was able to put his theory to the test. Around 1890, Wagner-Juaregg got his hands on something called tuberculin, a therapeutic treatment created by the German microbiologist Robert Koch in order to cure tuberculosis. Tuberculin would later turn out to be completely ineffective for treating tuberculosis, often creating severe immune responses in patients – but for a short time, Wagner-Juaregg had some success in using tuberculin to help his dementia patients. Giving his patients tuberculin resulted in a high fever – and after completing the treatment, Wagner-Jauregg reported that his patient’s dementia was completely halted. The success was short-lived, however: Wagner-Juaregg eventually had to discontinue tuberculin as a treatment, as it began to be considered too toxic.
By 1917, Wagner-Juaregg’s theory about syphilis and fevers was becoming more credible – and one day a new opportunity presented itself when a wounded soldier, stricken with malaria and a related fever, was accidentally admitted to his psychiatric unit.
When his findings were published in 1918, Wagner-Juaregg’s so-called “fever therapy” swept the globe.
What Wagner-Juaregg did next was ethically deplorable by any standard: Before he allowed the soldier any quinine (the standard treatment for malaria at the time), Wagner-Juaregg took a small sample of the soldier’s blood and inoculated three syphilis patients with the sample, rubbing the blood on their open syphilitic blisters.
It’s unclear how well the malaria treatment worked for those three specific patients – but Wagner-Juaregg’s records show that in the span of one year, he inoculated a total of nine patients with malaria, for the sole purpose of inducing fevers, and six of them made a full recovery. Wagner-Juaregg’s treatment was so successful, in fact, that one of his inoculated patients, an actor who was unable to work due to his dementia, was eventually able to find work again and return to the stage. Two additional patients – a military officer and a clerk – recovered from their once-terminal illnesses and returned to their former careers as well.
When his findings were published in 1918, Wagner-Juaregg’s so-called “fever therapy” swept the globe. The treatment was hailed as a breakthrough – but it still had risks. Malaria itself had a mortality rate of about 15 percent at the time. Many people considered that to be a gamble worth taking, compared to dying a painful, protracted death from syphilis.
Malaria could also be effectively treated much of the time with quinine, whereas other fever-causing illnesses were not so easily treated. Triggering a fever by way of malaria specifically, therefore, became the standard of care.
Tens of thousands of people with syphilitic dementia would go on to be treated with fever therapy until the early 1940s, when a combination of Salvarsan and penicillin caused syphilis infections to decline. Eventually, neurosyphilis became rare, and then nearly unheard of.
Despite his contributions to medicine, it’s important to note that Wagner-Juaregg was most definitely not a person to idolize. In fact, he was an outspoken anti-Semite and proponent of eugenics, arguing that Jews were more prone to mental illness and that people who were mentally ill should be forcibly sterilized. (Wagner-Juaregg later became a Nazi sympathizer during Hitler’s rise to power even though, bizarrely, his first wife was Jewish.) Another problematic issue was that his fever therapy involved experimental treatments on many who, due to their cognitive issues, could not give informed consent.
Lack of consent was also a fundamental problem with the syphilis study at Tuskegee, appalling research that began just 14 years after Wagner-Juaregg published his “fever therapy” findings.
Still, despite his outrageous views, Wagner-Juaregg was awarded the Nobel Prize in Medicine or Physiology in 1927 – and despite some egregious human rights abuses, the miraculous “fever therapy” was partly responsible for taming one of the deadliest plagues in human history.
An emerging technology called in vitro gametogenesis would allow babies to be made from skin cells.
[Ed. Note: This is the third episode in our Moonshot series, which will explore four cutting-edge scientific developments that stand to fundamentally transform our world.]
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.
My Wife's Fight Against Cancer Inspired 38,000 People to Raise Millions for Research
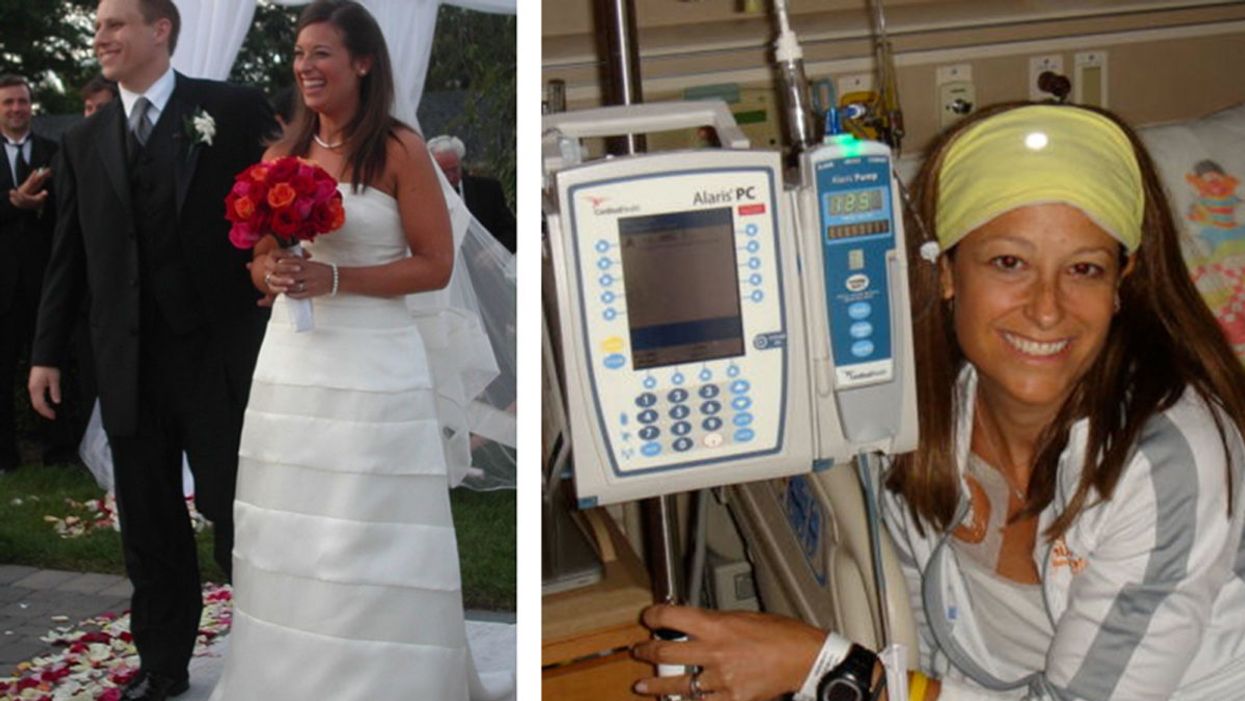
David and Jen on their wedding day, and Jen undergoing treatment for her rare sarcoma.
It was 15 years ago this month, but I'll never forget those words. When my wife Jen and I asked her oncologist about our plans to start a family, he calmly replied, "Well, I wouldn't do so unless Dave is prepared to be a single father."
About 50 percent of all people with cancer have a rare type, like the one Jen was fighting.
Time stood still. The danger crystalized — we were in a battle for my beautiful bride's life, and the odds were not in our favor.
We felt every emotion expected. Anger, sadness, confusion, frustration, and especially fear. But we made a very intentional choice to take that fear, put it to the side, and do everything we could to live our lives together to the fullest.
We focused first on Jen's health and learned everything we could about MFH Sarcoma. I was with her every step of the way — for hundreds of medical appointments, six intense surgeries, and twenty different types of chemotherapy. During such a challenging time, our choice to reject fear allowed us to live our best lives. Our careers blossomed, we enjoyed several international vacations, and Jen inspired thousands of fellow patients through her blog and speeches.
When we researched treatment options we learned that Jen was not alone. About 50 percent of all people with cancer have a rare type, like the one Jen was fighting. However, rare cancers don't get the funding they desperately need so effective treatment options are hard to find. The lack of funding felt unfair — and urgent. We didn't worry about everything that can go wrong when starting a new venture. Instead, we jumped in head first and convinced a small group of friends and family to ride stationary bikes with us to raise money for rare cancer research.

Jen Goodman Linn, riding a stationary bike for Cycle for Survival.
(Courtesy David Linn)
From those humble beginnings, Cycle for Survival grew steadily. After starting from scratch, Jen and I ran Cycle for Survival on our own for two years. We quickly realized that if we wanted to help as many people as possible, we needed the best partners. In 2009, we agreed that Memorial Sloan Kettering Cancer Center would take over the ownership of Cycle for Survival and Equinox officially became the Founding Partner. Flash forward to today, and Cycle for Survival has raised more than $220 million! I'm proud that 100% of every donation, yes every penny, goes directly into life-saving rare cancer research within six months of the annual indoor cycling events, which now take place in 17 cities nationwide.
While Cycle for Survival's trajectory was heading straight up, Jen's health struggle was devastatingly swinging up and down. With her incredible spirit and tenacity, Jen would beat the cancer through chemo and surgery, but then it would frustratingly come back again and again. After going into remission six times, it returned with such a vengeance in 2011 that even the world's leading doctors were forced to say, "I'm sorry, there's nothing more we can do."
Those were the most difficult words I've ever heard, by far. I hope no other family has to hear these crushing words.
When Jen died soon after, I didn't know what would happen to me, to my life, and to Cycle for Survival. I do remember making two very important choices at the time. First, I chose to get out of bed and put one foot in front of the other. It wasn't easy. Tears, pain, and grief would hit at any hour of the day or night. I did have a great support network of family and friends who kept me moving forward. One friend in particular changed the route of her morning runs so that I would join her and start getting back to exercising.
My second key choice was to stay involved with Cycle for Survival. At times, it was an excruciatingly difficult decision because I felt the depth of my loss each and every time I stepped into one of the events. However, it was also rewarding and energizing because I could see firsthand how many people it was helping, even though it was too late for Jen.
I began to travel across the country with the Cycle for Survival staff. My hope was to spread the word about rare cancers; along the way I met a lot of wonderful people who shared their stories with me. What I soon realized is that each of us faces obstacles in our lives. For me, it was losing the person who I wanted to spend my life with. For others, it might be challenges with their kids or in their professional lives. The common theme is that we don't have control over the fact that we have to face these challenges. But the biggest lesson I've learned is that we very much do have a choice in how we react.
I made the choice to do everything I can to help rare cancer patients and their families and it has been transformative and healing for me. The small group who rode in the first Cycle for Survival event has grown into a powerful movement of nearly 40,000 riders making a real difference. If Jen were diagnosed today, there are new treatments available– including genomic sequencing, targeted therapies, and immunotherapies – that could help her. Those weren't even options a short time ago. That's the result of funding research.

A recent Cycle for Survival event shows the passion and power of the community.
(Courtesy David Linn)
I also want to share one more choice I made. Remember that friend who changed the route of her morning runs so I could start exercising after Jen died? Well, over the years friendship grew into love, and we're now building a home together and can't wait to see what the future holds for us.
So with all that in mind I ask – when you face those inevitable challenges in your life, how will you choose to react? Remember that even in the midst of hopelessness, you can find choices. Those will be the decisions that define and guide you.