Trading syphilis for malaria: How doctors treated one deadly disease by infecting patients with another

In the 1920s, doctors induced a high fever in patients - so called "fever therapy" - as a way to help them recover from syphilis, though it involved ethical problems.
If you had lived one hundred years ago, syphilis – a bacterial infection spread by sexual contact – would likely have been one of your worst nightmares. Even though syphilis still exists, it can now be detected early and cured quickly with a course of antibiotics. Back then, however, before antibiotics and without an easy way to detect the disease, syphilis was very often a death sentence.
To understand how feared syphilis once was, it’s important to understand exactly what it does if it’s allowed to progress: the infections start off as small, painless sores or even a single sore near the vagina, penis, anus, or mouth. The sores disappear around three to six weeks after the initial infection – but untreated, syphilis moves into a secondary stage, often presenting as a mild rash in various areas of the body (such as the palms of a person’s hands) or through other minor symptoms. The disease progresses from there, often quietly and without noticeable symptoms, sometimes for decades before it reaches its final stages, where it can cause blindness, organ damage, and even dementia. Research indicates, in fact, that as much as 10 percent of psychiatric admissions in the early 20th century were due to dementia caused by syphilis, also known as neurosyphilis.
Like any bacterial disease, syphilis can affect kids, too. Though it’s spread primarily through sexual contact, it can also be transmitted from mother to child during birth, causing lifelong disability.
The poet-physician Aldabert Bettman, who wrote fictionalized poems based on his experiences as a doctor in the 1930s, described the effect syphilis could have on an infant in his poem Daniel Healy:
I always got away clean
when I went out
With the boys.
The night before
I was married
I went out,—But was not so fortunate;
And I infected
My bride.
When little Daniel
Was born
His eyes discharged;
And I dared not tell
That because
I had seen too much
Little Daniel sees not at all
Given the horrors of untreated syphilis, it’s maybe not surprising that people would go to extremes to try and treat it. One of the earliest remedies for syphilis, dating back to 15th century Naples, was using mercury – either rubbing it on the skin where blisters appeared, or breathing it in as a vapor. (Not surprisingly, many people who underwent this type of “treatment” died of mercury poisoning.)
Other primitive treatments included using tinctures made of a flowering plant called guaiacum, as well as inducing “sweat baths” to eliminate the syphilitic toxins. In 1910, an arsenic-based drug called Salvarsan hit the market and was hailed as a “magic bullet” for its ability to target and destroy the syphilis-causing bacteria without harming the patient. However, while Salvarsan was effective in treating early-stage syphilis, it was largely ineffective by the time the infection progressed beyond the second stage. Tens of thousands of people each year continued to die of syphilis or were otherwise shipped off to psychiatric wards due to neurosyphilis.
It was in one of these psychiatric units in the early 20th century that Dr. Julius Wagner-Juaregg got the idea for a potential cure.
Wagner-Juaregg was an Austrian-born physician trained in “experimental pathology” at the University of Vienna. Wagner-Juaregg started his medical career conducting lab experiments on animals and then moved on to work at different psychiatric clinics in Vienna, despite having no training in psychiatry or neurology.
Wagner-Juaregg’s work was controversial to say the least. At the time, medicine – particularly psychiatric medicine – did not have anywhere near the same rigorous ethical standards that doctors, researchers, and other scientists are bound to today. Wagner-Juaregg would devise wild theories about the cause of their psychiatric ailments and then perform experimental procedures in an attempt to cure them. (As just one example, Wagner-Juaregg would sterilize his adolescent male patients, thinking “excessive masturbation” was the cause of their schizophrenia.)
But sometimes these wild theories paid off. In 1883, during his residency, Wagner-Juaregg noted that a female patient with mental illness who had contracted a skin infection and suffered a high fever experienced a sudden (and seemingly miraculous) remission from her psychosis symptoms after the fever had cleared. Wagner-Juaregg theorized that inducing a high fever in his patients with neurosyphilis could help them recover as well.
Eventually, Wagner-Juaregg was able to put his theory to the test. Around 1890, Wagner-Juaregg got his hands on something called tuberculin, a therapeutic treatment created by the German microbiologist Robert Koch in order to cure tuberculosis. Tuberculin would later turn out to be completely ineffective for treating tuberculosis, often creating severe immune responses in patients – but for a short time, Wagner-Juaregg had some success in using tuberculin to help his dementia patients. Giving his patients tuberculin resulted in a high fever – and after completing the treatment, Wagner-Jauregg reported that his patient’s dementia was completely halted. The success was short-lived, however: Wagner-Juaregg eventually had to discontinue tuberculin as a treatment, as it began to be considered too toxic.
By 1917, Wagner-Juaregg’s theory about syphilis and fevers was becoming more credible – and one day a new opportunity presented itself when a wounded soldier, stricken with malaria and a related fever, was accidentally admitted to his psychiatric unit.
When his findings were published in 1918, Wagner-Juaregg’s so-called “fever therapy” swept the globe.
What Wagner-Juaregg did next was ethically deplorable by any standard: Before he allowed the soldier any quinine (the standard treatment for malaria at the time), Wagner-Juaregg took a small sample of the soldier’s blood and inoculated three syphilis patients with the sample, rubbing the blood on their open syphilitic blisters.
It’s unclear how well the malaria treatment worked for those three specific patients – but Wagner-Juaregg’s records show that in the span of one year, he inoculated a total of nine patients with malaria, for the sole purpose of inducing fevers, and six of them made a full recovery. Wagner-Juaregg’s treatment was so successful, in fact, that one of his inoculated patients, an actor who was unable to work due to his dementia, was eventually able to find work again and return to the stage. Two additional patients – a military officer and a clerk – recovered from their once-terminal illnesses and returned to their former careers as well.
When his findings were published in 1918, Wagner-Juaregg’s so-called “fever therapy” swept the globe. The treatment was hailed as a breakthrough – but it still had risks. Malaria itself had a mortality rate of about 15 percent at the time. Many people considered that to be a gamble worth taking, compared to dying a painful, protracted death from syphilis.
Malaria could also be effectively treated much of the time with quinine, whereas other fever-causing illnesses were not so easily treated. Triggering a fever by way of malaria specifically, therefore, became the standard of care.
Tens of thousands of people with syphilitic dementia would go on to be treated with fever therapy until the early 1940s, when a combination of Salvarsan and penicillin caused syphilis infections to decline. Eventually, neurosyphilis became rare, and then nearly unheard of.
Despite his contributions to medicine, it’s important to note that Wagner-Juaregg was most definitely not a person to idolize. In fact, he was an outspoken anti-Semite and proponent of eugenics, arguing that Jews were more prone to mental illness and that people who were mentally ill should be forcibly sterilized. (Wagner-Juaregg later became a Nazi sympathizer during Hitler’s rise to power even though, bizarrely, his first wife was Jewish.) Another problematic issue was that his fever therapy involved experimental treatments on many who, due to their cognitive issues, could not give informed consent.
Lack of consent was also a fundamental problem with the syphilis study at Tuskegee, appalling research that began just 14 years after Wagner-Juaregg published his “fever therapy” findings.
Still, despite his outrageous views, Wagner-Juaregg was awarded the Nobel Prize in Medicine or Physiology in 1927 – and despite some egregious human rights abuses, the miraculous “fever therapy” was partly responsible for taming one of the deadliest plagues in human history.
Electricity is emerging as a powerful treatment for chronic ailments.
Kelly, a case manager for an insurance company, spent years battling both migraines and Crohn's, a disease in which the immune system attacks the intestines.
For many people, like Kelly, a stronger electric boost to the vagus nerve could be life-changing.
After she had her large intestine removed, her body couldn't absorb migraine medication. Last year, about twice a month, she endured migraines so bad she couldn't function. "It would go up to a ten, and I would rock, wait it out," she said. The pain might last for three days.
Then her neurologist showed her a new device, gammaCore, that tames migraines by stimulating a nerve—not medication. "I don't have to put a chemical in my body," she said. "I was thrilled."
At first, Kelly used the device at the onset of a migraine, applying electricity to her pulse at the front of her neck for six minutes. The pain peaked at about half the usual intensity--low enough, she said, that she could go to work. Four months ago, she began using the device for two minutes each night as prevention, and she hasn't had a serious migraine since.
The Department of Defense and Veterans Administration now offer gammaCore to patients, but it hasn't yet been approved by Medicare, Medicaid, or most insurers. A month of therapy costs $600 before insurance or a generous financial assistance program kicks in.

A patient uses gammaCore, a non invasive vagal nerve stimulator device that was FDA approved in November 2018, to treat her migraine.
(Photo captured from a patient video at gammacore.com)
If the poet Walt Whitman wrote "I Sing The Body Electric" today, he might get specific and point to the vagus nerve, a bundle of fibers that run from the brainstem down the neck to the heart and gut. Singing stimulates it—and for many people, like Kelly, a stronger electric boost to the nerve could be life-changing.
The mind-body connection isn't just an idea — the vagus nerve literally carries signals from the mind to the body and back. It may explain the link between childhood trauma and illnesses such as chronic pain and headaches in adults. "How is it possible that a psychological event causes pain years later?" asked Peter Staats, co-founder of electroCore, which has won approval for its new device from the Food and Drug Administration (FDA) for both migraine and cluster headaches. "There has to be a mind-body interface, and that is the vagus nerve," he said.
Scientists knew that this nerve controlled your heart rate and blood pressure, but in the past decade it has been linked to both pain and the immune system.
"Everything is gated through the vagus -- problems with the gut, the heart, and the lungs," said Chris Wilson, a researcher at Loma Linda University, in California. Wilson is studying how vagus nerve stimulation (VNS) could help pre-term babies who develop lung infections. "Nearly every one of our chronic diseases, including cancer, Alzheimer's, Parkinson's, chronic arthritis and rheumatoid arthritis, and depression and chronic pain…could benefit from an appropriate stimulator," he said.
It's unfortunate that Kelly got her device only after her large intestine was gone. SetPoint Medical, a privately held California company founded to develop electronic treatments for chronic autoimmune diseases, has announced early positive results with VNS for both Crohn's and rheumatoid arthritis.
As SetPoint's chief medical officer, David Chernoff, put it, "We're hacking into the nervous system to activate a system that is already there," an approach that, he said, could work "on many diseases that are pain- and inflammation-based." Inflammation plays a role in much modern illness, including depression and obesity. The FDA already has approved VNS for both, using surgically implanted devices similar to pacemakers. (GammaCore is external.)
The history of VNS implants goes back to 1997, when the FDA approved one for treating epilepsy and researchers noticed that it rapidly lifted depression in epileptic patients. By 2005, the agency had approved an implant for treatment-resistant depression. (Insurance companies declined to reimburse the approach and it didn't take off, but that might change: in February, the Center for Medicare and Medicaid Services asked for more data to evaluate coverage.) In 2015, the FDA approved an implant in the abdomen to regulate appetite signals and help obese people lose weight.
The link to inflammation had emerged a decade earlier, when researchers at the Feinstein Institute for Medical Research, in Manhasset, New York, demonstrated that stimulating the nerve with electricity in rats suppressed the production of cytokines, a signaling protein important in the immune system. The researchers developed a concept of a hard-wired pathway, through the vagus nerve, between the immune and nervous system. That pathway, they argued, regulates inflammation. While other researchers argue that VNS is helpful by other routes, there is clear evidence that, one way or another, it does affect immunity.
At the same time, investors are seeking alternatives to drugs.
The Feinstein rat research concluded that it took only a minute a day of stimulation and tiny amounts of energy to activate an anti-inflammatory reflex. This means you can use devices "the size of a coffee bean," said Chernoff, much less clunky than current pacemakers—and advances in electronic technology are making them possible.
At the same time, investors are seeking alternatives to drugs. "There's been a push back on drug pricing," noted Lisa Rhoads, a managing director at Easton Capital Investment Group, in New York, which supported electroCore, "and so many unintended consequences."
In 2016, the U.S. National Institutes of Health began pumping money into relevant research, in a program called "Stimulating Peripheral Activity to Relieve Conditions," which focuses on "understanding peripheral nerves — nerves that connect the brain and spinal cord to the rest of the body — and how their electrical signals control internal organ function."
GlaxoSmithKline formed Galvani Bioelectronics with Google to study miniature implants. It had already invested in Action Potential Venture Capital, in Cambridge, Massachusetts, which holds SetPoint and seven other companies "that are all targeting a nerve to treat a chronic disease," noted partner Imran Eba. "I see a future in which bioelectronics medicine is competing directly with drugs," he said.
Treating the body with electricity could bring more ease and lower costs. Many people with serious auto-immune disease, for example, have to inject themselves with drugs that cost $60,000 a year. SetPoint's implant would cost less and only need charging once a week, using a charger worn around the neck, Chernoff said. The company receives notices remotely and can monitor compliance.
Implants also allow the treatment to target a nerve precisely, which could be important with Parkinson's, chronic pain, and depression, observed James Cavuoto, editor and publisher of Neurotech Reports. They may also allow for more fine-turning. "In general, the industry is looking for signals, biomarkers that indicate when is the right time to turn on and turn off the stimulation. It could dramatically increase the effectiveness of the therapy and conserve battery life," he said.
Eventually, external devices could receive data from biomarkers as well. "It could be something you wear on your wrist," Cavuoto noted. Bluetooth-enabled devices could communicate with phones or laptops for data capture. External devices don't require surgery and put the patient in charge. "In the future you'll see more customer specification: Give the patient a tablet or phone app that lets them track and modify their parameters, within a range. With digital devices we have an enormous capability to customize therapies and collect data and get feedback that can be fed back to the clinician," Cavuoto said.
Slow deep breathing, the traditional mind-body intervention, is "like watching Little League. What we're doing is Major League."
It's even possible to stimulate the vagus through the ear, where one branch of the bundle of fibers begins. In a fetus, the tissue that becomes the ear is also part of the vagus nerve, and that one bit remains. "It's the same point as the acupuncture point," explained Mark George, a psychiatrist and pioneer researcher in depression at Medical University of South Carolina in Charleston. "Acupuncture figured out years ago by trial and error what we're just learning about now."
Slow deep breathing, the traditional mind-body intervention, also affects the vagus nerve in positive ways, but gently. "That's like watching Little League," Staats, the co-founder of electroCore, said. "What we're doing is Major League."
In ten years, researcher Wilson suggested, you could be wearing "a little ear cuff" that monitors your basic autonomic tone, a heart-attack risk measure governed in part by the vagus nerve. If your tone looked iffy, the stimulator would intervene, he said, "and improve your mood, cognition, and health."
In the meantime, we can take some long slow breaths, read Whitman, and sing.
“Siri, Read My Mind”: A New Device Lets Users Think Commands
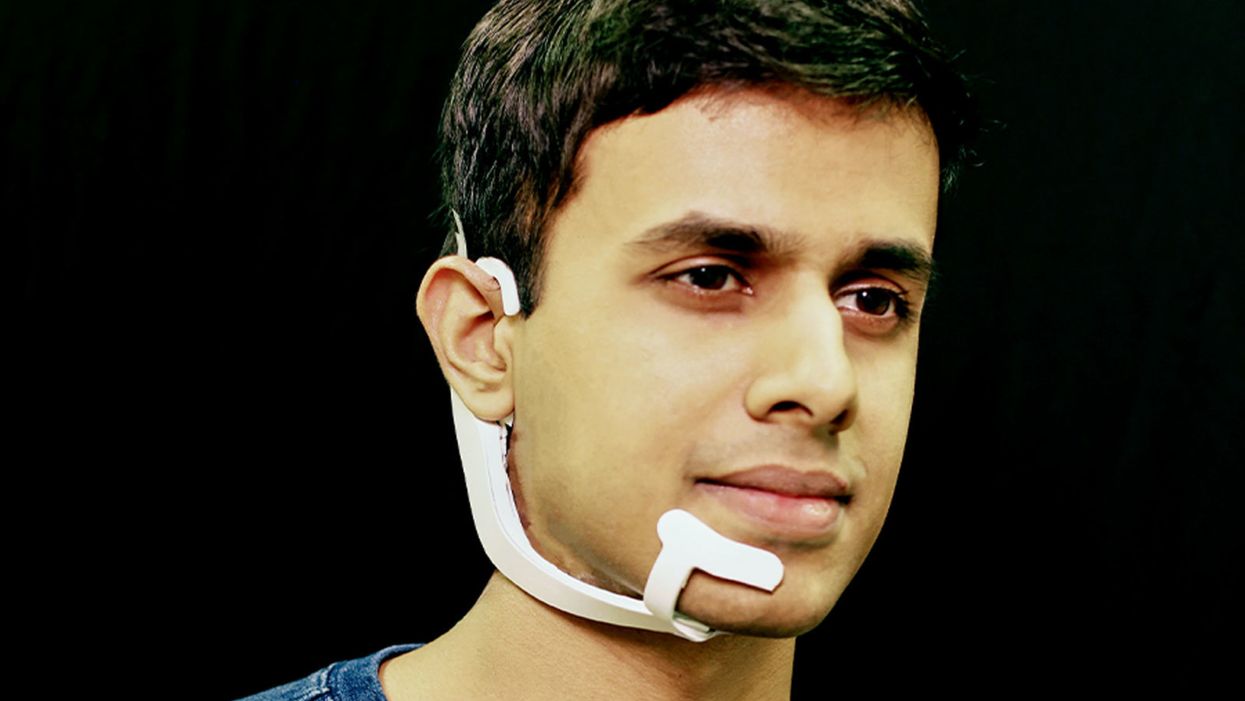
Arnav Kapur, a researcher in the Fluid Interfaces Group at MIT, wears an earlier prototype of the AlterEgo. A newer version is more discreet.
Sometime in the near future, we won't need to type on a smartphone or computer to silently communicate our thoughts to others.
"We're moving as fast as possible to get the technology right, to get the ethics right, to get everything right."
In fact, the devices themselves will quietly understand our intentions and express them to other people. We won't even need to move our mouths.
That "sometime in the near future" is now.
At the recent TED Conference, MIT student and TED Fellow Arnav Kapur was onstage with a colleague doing the first live public demo of his new technology. He was showing how you can communicate with a computer using signals from your brain. The usually cool, erudite audience seemed a little uncomfortable.
"If you look at the history of computing, we've always treated computers as external devices that compute and act on our behalf," Kapur said. "What I want to do is I want to weave computing, AI and Internet as part of us."
His colleague started up a device called AlterEgo. Thin like a sticker, AlterEgo picks up signals in the mouth cavity. It recognizes the intended speech and processes it through the built-in AI. The device then gives feedback to the user directly through bone conduction: It vibrates your inner ear drum and gives you a response meshing with your normal hearing.
Onstage, the assistant quietly thought of a question: "What is the weather in Vancouver?" Seconds later, AlterEgo told him in his ear. "It's 50 degrees and rainy here in Vancouver," the assistant announced.
AlterEgo essentially gives you a built-in Siri.
"We don't have a deadline [to go to market], but we're moving as fast as possible to get the technology right, to get the ethics right, to get everything right," Kapur told me after the talk. "We're developing it both as a general purpose computer interface and [in specific instances] like on the clinical side or even in people's homes."
Nearly-telepathic communication actually makes sense now. About ten years ago, the Apple iPhone replaced the ubiquitous cell phone keyboard with a blank touchscreen. A few years later, Google Glass put computer screens into a simple lens. More recently, Amazon Alexa and Microsoft Cortana have dropped the screen and gone straight for voice control. Now those voices are getting closer to our minds and may even become indistinguishable in the future.
"We knew the voice market was growing, like with getting map locations, and audio is the next frontier of user interfaces," says Dr. Rupal Patel, Founder and CEO of VocalID. The startup literally gives voices to the voiceless, particularly people unable to speak because of illness or other circumstances.
"We start with [our database of] human voices, then train our deep learning technology to learn the pattern of speech… We mix voices together from our voice bank, so it's not just Damon's voice, but three or five voices. They are different enough to blend it into a voice that does not exist today – kind of like a face morph."
The VocalID customer then has a voice as unique as he or she is, mixed together like a Sauvignon blend. It is a surrogate voice for those of us who cannot speak, just as much as AlterEgo is a surrogate companion for our brains.
"I'm very skeptical keyboards or voice-based communication will be replaced any time soon."
Voice equality will become increasingly important as Siri, Alexa and voice-based interfaces become the dominant communication method.
It may feel odd to view your voice as a privilege, but as the world becomes more voice-activated, there will be a wider gap between the speakers and the voiceless. Picture going shopping without access to the Internet or trying to eat healthily when your neighborhood is a food desert. And suffering from vocal difficulties is more common than you might think. In fact, according to government statistics, around 7.5 million people in the U.S. have trouble using their voices.
While voice communication appears to be here to stay, at least for now, a more radical shift to mind-controlled communication is not necessarily inevitable. Tech futurist Wagner James Au, for one, is dubious.
"I'm very skeptical keyboards or voice-based communication will be replaced any time soon. Generation Z has grown up with smartphones and games like Fortnite, so I don't see them quickly switching to a new form factor. It's still unclear if even head-mounted AR/VR displays will see mass adoption, and mind-reading devices are a far greater physical imposition on the user."
How adopters use the newest brain impulse-reading, voice-altering technology is a much more complicated discussion. This spring, a video showed U.S. House Speaker Nancy Pelosi stammering and slurring her words at a press conference. The problem is that it didn't really happen: the video was manufactured and heavily altered from the original source material.
So-called deepfake videos use computer algorithms to capture the visual and vocal cues of an individual, and then the creator can manipulate it to say whatever it wants. Deepfakes have already created false narratives in the political and media systems – and these are only videos. Newer tech is making the barrier between tech and our brains, if not our entire identity, even thinner.
"Last year," says Patel of VocalID, "we did penetration testing with our voices on banks that use voice control – and our generation 4 system is even tricky for you and me to identify the difference (between real and fake). As a forward-thinking company, we want to prevent risk early on by watermarking voices, creating a detector of false voices, and so on." She adds, "The line will become more blurred over time."
Onstage at TED, Kapur reassured the audience about who would be in the driver's seat. "This is why we designed the system to deliberately record from the peripheral nervous system, which is why the control in all situations resides with the user."
And, like many creators, he quickly shifted back to the possibilities. "What could the implications of something like this be? Imagine perfectly memorizing things, where you perfectly record information that you silently speak, and then hear them later when you want to, internally searching for information, crunching numbers at speeds computers do, silently texting other people."
"The potential," he concluded, "could be far-reaching."