Catching colds may help protect kids from Covid

A new study shows that the immune system's response to colds can help prepare it to defend against COVID-19 - but only in the very young.
A common cold virus causes the immune system to produce T cells that also provide protection against SARS-CoV-2, according to new research. The study, published last month in PNAS, shows that this effect is most pronounced in young children. The finding may help explain why most young people who have been exposed to the cold-causing coronavirus have not developed serious cases of COVID-19.
One curiosity stood out in the early days of the COVID-19 pandemic – why were so few kids getting sick. Generally young children and the elderly are the most vulnerable to disease outbreaks, particularly viral infections, either because their immune systems are not fully developed or they are starting to fail.
But solid information on the new infection was so scarce that many public health officials acted on the precautionary principle, assumed a worst-case scenario, and applied the broadest, most restrictive policies to all people to try to contain the coronavirus SARS-CoV-2.
One early thought was that lockdowns worked and kids (ages 6 months to 17 years) simply were not being exposed to the virus. So it was a shock when data started to come in showing that well over half of them carried antibodies to the virus, indicating exposure without getting sick. That trend grew over time and the latest tracking data from the CDC shows that 96.3 percent of kids in the U.S. now carry those antibodies.
Antibodies are relatively quick and easy to measure, but some scientists are exploring whether the reactions of T cells could serve as a more useful measure of immune protection.
But that couldn't be the whole story because antibody protection fades, sometimes as early as a month after exposure and usually within a year. Additionally, SARS-CoV-2 has been spewing out waves of different variants that were more resistant to antibodies generated by their predecessors. The resistance was so significant that over time the FDA withdrew its emergency use authorization for a handful of monoclonal antibodies with earlier approval to treat the infection because they no longer worked.
Antibodies got most of the attention early on because they are part of the first line response of the immune system. Antibodies can bind to viruses and neutralize them, preventing infection. They are relatively quick and easy to measure and even manufacture, but as SARS-CoV-2 showed us, often viruses can quickly evolve to become more resistant to them. Some scientists are exploring whether the reactions of T cells could serve as a more useful measure of immune protection.
Kids, colds and T cells
T cells are part of the immune system that deals with cells once they have become infected. But working with T cells is much more difficult, takes longer, and is more expensive than working with antibodies. So studies often lags behind on this part of the immune system.
A group of researchers led by Annika Karlsson at the Karolinska Institute in Sweden focuses on T cells targeting virus-infected cells and, unsurprisingly, saw that they can play a role in SARS-CoV-2 infection. Other labs have shown that vaccination and natural exposure to the virus generates different patterns of T cell responses.
The Swedes also looked at another member of the coronavirus family, OC43, which circulates widely and is one of several causes of the common cold. The molecular structure of OC43 is similar to its more deadly cousin SARS-CoV-2. Sometimes a T cell response to one virus can produce a cross-reactive response to a similar protein structure in another virus, meaning that T cells will identify and respond to the two viruses in much the same way. Karlsson looked to see if T cells for OC43 from a wide age range of patients were cross-reactive to SARS-CoV-2.
And that is what they found, as reported in the PNAS study last month; there was cross-reactive activity, but it depended on a person’s age. A subset of a certain type of T cells, called mCD4+,, that recognized various protein parts of the cold-causing virus, OC43, expressed on the surface of an infected cell – also recognized those same protein parts from SARS-CoV-2. The T cell response was lower than that generated by natural exposure to SARS-CoV-2, but it was functional and thus could help limit the severity of COVID-19.
“One of the most politicized aspects of our pandemic response was not accepting that children are so much less at risk for severe disease with COVID-19,” because usually young children are among the most vulnerable to pathogens, says Monica Gandhi, professor of medicine at the University of California San Francisco.
“The cross-reactivity peaked at age six when more than half the people tested have a cross-reactive immune response,” says Karlsson, though their sample is too small to say if this finding applies more broadly across the population. The vast majority of children as young as two years had OC43-specific mCD4+ T cell responses. In adulthood, the functionality of both the OC43-specific and the cross-reactive T cells wane significantly, especially with advanced age.
“Considering that the mortality rate in children is the lowest from ages five to nine, and higher in younger children, our results imply that cross-reactive mCD4+ T cells may have a role in the control of SARS-CoV-2 infection in children,” the authors wrote in their paper.
“One of the most politicized aspects of our pandemic response was not accepting that children are so much less at risk for severe disease with COVID-19,” because usually young children are among the most vulnerable to pathogens, says Monica Gandhi, professor of medicine at the University of California San Francisco and author of the book, Endemic: A Post-Pandemic Playbook, to be released by the Mayo Clinic Press this summer. The immune response of kids to SARS-CoV-2 stood our expectations on their head. “We just haven't seen this before, so knowing the mechanism of protection is really important.”
Why the T cell immune response can fade with age is largely unknown. With some viruses such as measles, a single vaccination or infection generates life-long protection. But respiratory tract infections, like SARS-CoV-2, cause a localized infection - specific to certain organs - and that response tends to be shorter lived than systemic infections that affect the entire body. Karlsson suspects the elderly might be exposed to these localized types of viruses less often. Also, frequent continued exposure to a virus that results in reactivation of the memory T cell pool might eventually result in “a kind of immunosenescence or immune exhaustion that is associated with aging,” Karlsson says. https://leaps.org/scientists-just-started-testing-a-new-class-of-drugs-to-slow-and-even-reverse-aging/particle-3 This fading protection is why older people need to be repeatedly vaccinated against SARS-CoV-2.
Policy implications
Following the numbers on COVID-19 infections and severity over the last three years have shown us that healthy young people without risk factors are not likely to develop serious disease. This latest study points to a mechanism that helps explain why. But the inertia of existing policies remains. How should we adjust policy recommendations based on what we know today?
The World Health Organization (WHO) updated their COVID-19 vaccination guidance on March 28. It calls for a focus on vaccinating and boosting those at risk for developing serious disease. The guidance basically shrugged its shoulders when it came to healthy children and young adults receiving vaccinations and boosters against COVID-19. It said the priority should be to administer the “traditional essential vaccines for children,” such as those that protect against measles, rubella, and mumps.
“As an immunologist and a mother, I think that catching a cold or two when you are a kid and otherwise healthy is not that bad for you. Children have a much lower risk of becoming severely ill with SARS-CoV-2,” says Karlsson. She has followed public health guidance in Sweden, which means that her young children have not been vaccinated, but being older, she has received the vaccine and boosters. Gandhi and her children have been vaccinated, but they do not plan on additional boosters.
The WHO got it right in “concentrating on what matters,” which is getting traditional childhood immunizations back on track after their dramatic decline over the last three years, says Gandhi. Nor is there a need for masking in schools, according to a study from the Catalonia region of Spain. It found “no difference in masking and spread in schools,” particularly since tracking data indicate that nearly all young people have been exposed to SARS-CoV-2.
Both researchers lament that public discussion has overemphasized the quickly fading antibody part of the immune response to SARS-CoV-2 compared with the more durable T cell component. They say developing an efficient measure of T cell response for doctors to use in the clinic would help to monitor immunity in people at risk for severe cases of COVID-19 compared with the current method of toting up potential risk factors.
After spaceflight record, NASA looks to protect astronauts on even longer trips
NASA astronaut Frank Rubio floats by the International Space Station’s “window to the world.” Yesterday, he returned from the longest single spaceflight by a U.S. astronaut on record - over one year. Exploring deep space will require even longer missions.
At T-minus six seconds, the main engines of the Atlantis Space Shuttle ignited, rattling its capsule “like a skyscraper in an earthquake,” according to astronaut Tom Jones, describing the 1988 launch. As the rocket lifted off and accelerated to three times the force of Earth's gravity, “It felt as if two of my friends were standing on my chest and wouldn’t get off.” But when Atlantis reached orbit, the main engines cut off, and the astronauts were suddenly weightless.
Since 1961, NASA has sent hundreds of astronauts into space while working to making their voyages safer and smoother. Yet, challenges remain. Weightlessness may look amusing when watched from Earth, but it has myriad effects on cognition, movement and other functions. When missions to space stretch to six months or longer, microgravity can impact astronauts’ health and performance, making it more difficult to operate their spacecraft.
Yesterday, NASA astronaut Frank Rubio returned to Earth after over one year, the longest single spaceflight for a U.S. astronaut. But this is just the start; longer and more complex missions into deep space loom ahead, from returning to the moon in 2025 to eventually sending humans to Mars. To ensure that these missions succeed, NASA is increasing efforts to study the biological effects and prevent harm.
The dangers of microgravity are real
A NASA report published in 2016 details a long list of incidents and near-misses caused – at least partly – by space-induced changes in astronauts’ vision and coordination. These issues make it harder to move with precision and to judge distance and velocity.
According to the report, in 1997, a resupply ship collided with the Mir space station, possibly because a crew member bumped into the commander during the final docking maneuver. This mishap caused significant damage to the space station.
Returns to Earth suffered from problems, too. The same report notes that touchdown speeds during the first 100 space shuttle landings were “outside acceptable limits. The fastest landing on record – 224 knots (258 miles) per hour – was linked to the commander’s momentary spatial disorientation.” Earlier, each of the six Apollo crews that landed on the moon had difficulty recognizing moon landmarks and estimating distances. For example, Apollo 15 landed in an unplanned area, ultimately straddling the rim of a five-foot deep crater on the moon, harming one of its engines.
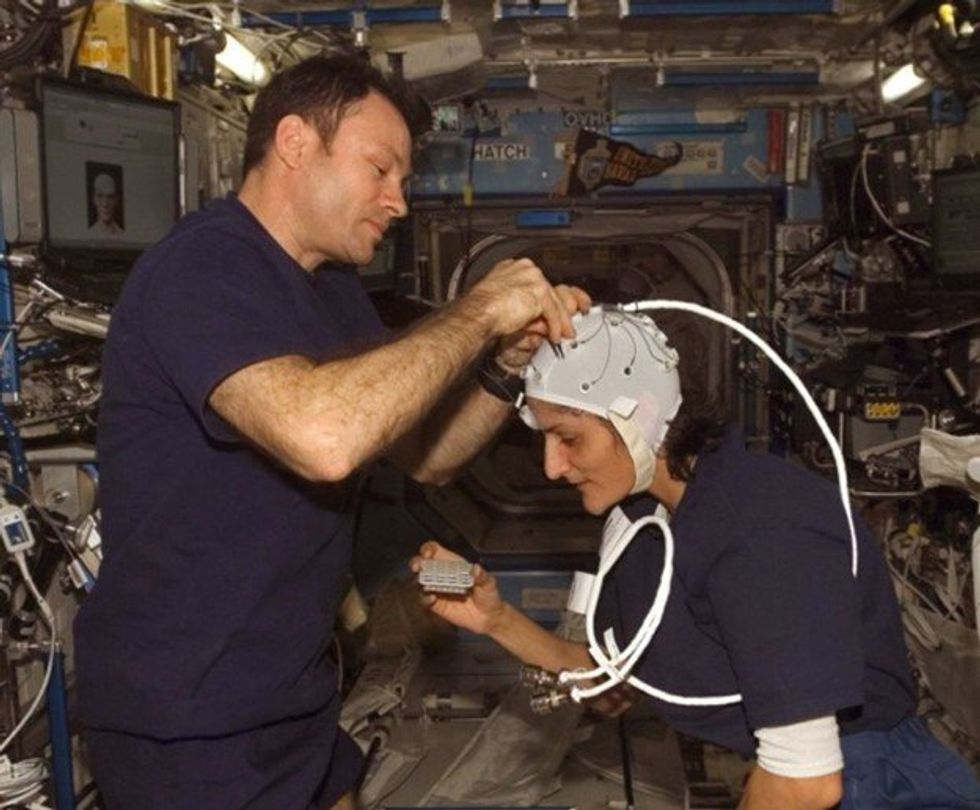
Spaceflight causes unique stresses on astronauts’ brains and central nervous systems. NASA is working to reduce these harmful effects.
NASA
Space messes up your brain
In space, astronauts face the challenges of microgravity, ionizing radiation, social isolation, high workloads, altered circadian rhythms, monotony, confined living quarters and a high-risk environment. Among these issues, microgravity is one of the most consequential in terms of physiological changes. It changes the brain’s structure and its functioning, which can hurt astronauts’ performance.
The brain shifts upwards within the skull, displacing the cerebrospinal fluid, which reduces the brain’s cushioning. Essentially, the brain becomes crowded inside the skull like a pair of too-tight shoes.
That’s partly because of how being in space alters blood flow. On Earth, gravity pulls our blood and other internal fluids toward our feet, but our circulatory valves ensure that the fluids are evenly distributed throughout the body. In space, there’s not enough gravity to pull the fluids down, and they shift up, says Rachael D. Seidler, a physiologist specializing in spaceflight at the University of Florida and principal investigator on many space-related studies. The head swells and legs appear thinner, causing what astronauts call “puffy face chicken legs.”
“The brain changes at the structural and functional level,” says Steven Jillings, equilibrium and aerospace researcher at the University of Antwerp in Belgium. “The brain shifts upwards within the skull,” displacing the cerebrospinal fluid, which reduces the brain’s cushioning. Essentially, the brain becomes crowded inside the skull like a pair of too-tight shoes. Some of the displaced cerebrospinal fluid goes into cavities within the brain, called ventricles, enlarging them. “The remaining fluids pool near the chest and heart,” explains Jillings. After 12 consecutive months in space, one astronaut had a ventricle that was 25 percent larger than before the mission.
Some changes reverse themselves while others persist for a while. An example of a longer-lasting problem is spaceflight-induced neuro-ocular syndrome, which results in near-sightedness and pressure inside the skull. A study of approximately 300 astronauts shows near-sightedness affects about 60 percent of astronauts after long missions on the International Space Station (ISS) and more than 25 percent after spaceflights of only a few weeks.
Another long-term change could be the decreased ability of cerebrospinal fluid to clear waste products from the brain, Seidler says. That’s because compressing the brain also compresses its waste-removing glymphatic pathways, resulting in inflammation, vulnerability to injuries and worsening its overall health.
The effects of long space missions were best demonstrated on astronaut twins Scott and Mark Kelly. This NASA Twins Study showed multiple, perhaps permanent, changes in Scott after his 340-day mission aboard the ISS, compared to Mark, who remained on Earth. The differences included declines in Scott’s speed, accuracy and cognitive abilities that persisted longer than six months after returning to Earth in March 2016.
By the end of 2020, Scott’s cognitive abilities improved, but structural and physiological changes to his eyes still remained, he said in a BBC interview.
“It seems clear that the upward shift of the brain and compression of the surrounding tissues with ventricular expansion might not be a good thing,” Seidler says. “But, at this point, the long-term consequences to brain health and human performance are not really known.”

NASA astronaut Kate Rubins conducts a session for the Neuromapping investigation.
NASA
Staying sharp in space
To investigate how prolonged space travel affects the brain, NASA launched a new initiative called the Complement of Integrated Protocols for Human Exploration Research (CIPHER). “CIPHER investigates how long-duration spaceflight affects both brain structure and function,” says neurobehavioral scientist Mathias Basner at the University of Pennsylvania, a principal investigator for several NASA studies. “Through it, we can find out how the brain adapts to the spaceflight environment and how certain brain regions (behave) differently after – relative to before – the mission.”
To do this, he says, “Astronauts will perform NASA’s cognition test battery before, during and after six- to 12-month missions, and will also perform the same test battery in an MRI scanner before and after the mission. We have to make sure we better understand the functional consequences of spaceflight on the human brain before we can send humans safely to the moon and, especially, to Mars.”
As we go deeper into space, astronauts cognitive and physical functions will be even more important. “A trip to Mars will take about one year…and will introduce long communication delays,” Seidler says. “If you are on that mission and have a problem, it may take eight to 10 minutes for your message to reach mission control, and another eight to 10 minutes for the response to get back to you.” In an emergency situation, that may be too late for the response to matter.
“On a mission to Mars, astronauts will be exposed to stressors for unprecedented amounts of time,” Basner says. To counter them, NASA is considering the continuous use of artificial gravity during the journey, and Seidler is studying whether artificial gravity can reduce the harmful effects of microgravity. Some scientists are looking at precision brain stimulation as a way to improve memory and reduce anxiety due to prolonged exposure to radiation in space.
Other scientists are exploring how to protect neural stem cells (which create brain cells) from radiation damage, developing drugs to repair damaged brain cells and protect cells from radiation.
To boldly go where no astronauts have gone before, they must have optimal reflexes, vision and decision-making. In the era of deep space exploration, the brain—without a doubt—is the final frontier.
Additionally, NASA is scrutinizing each aspect of the mission, including astronaut exercise, nutrition and intellectual engagement. “We need to give astronauts meaningful work. We need to stimulate their sensory, cognitive and other systems appropriately,” Basner says, especially given their extreme confinement and isolation. The scientific experiments performed on the ISS – like studying how microgravity affects the ability of tissue to regenerate is a good example.
“We need to keep them engaged socially, too,” he continues. The ISS crew, for example, regularly broadcasts from space and answers prerecorded questions from students on Earth, and can engage with social media in real time. And, despite tight quarters, NASA is ensuring the crew capsule and living quarters on the moon or Mars include private space, which is critical for good mental health.
Exploring deep space builds on a foundation that began when astronauts first left the planet. With each mission, scientists learn more about spaceflight effects on astronauts’ bodies. NASA will be using these lessons to succeed with its plans to build science stations on the moon and, eventually, Mars.
“Through internally and externally led research, investigations implemented in space and in spaceflight simulations on Earth, we are striving to reduce the likelihood and potential impacts of neurostructural changes in future, extended spaceflight,” summarizes NASA scientist Alexandra Whitmire. To boldly go where no astronauts have gone before, they must have optimal reflexes, vision and decision-making. In the era of deep space exploration, the brain—without a doubt—is the final frontier.
A newly discovered brain cell may lead to better treatments for cognitive disorders
Swiss researchers have found a type of brain cell that appears to be a hybrid of the two other main types — and it could lead to new treatments for brain disorders.
Swiss researchers have discovered a third type of brain cell that appears to be a hybrid of the two other primary types — and it could lead to new treatments for many brain disorders.
The challenge: Most of the cells in the brain are either neurons or glial cells. While neurons use electrical and chemical signals to send messages to one another across small gaps called synapses, glial cells exist to support and protect neurons.
Astrocytes are a type of glial cell found near synapses. This close proximity to the place where brain signals are sent and received has led researchers to suspect that astrocytes might play an active role in the transmission of information inside the brain — a.k.a. “neurotransmission” — but no one has been able to prove the theory.
A new brain cell: Researchers at the Wyss Center for Bio and Neuroengineering and the University of Lausanne believe they’ve definitively proven that some astrocytes do actively participate in neurotransmission, making them a sort of hybrid of neurons and glial cells.
According to the researchers, this third type of brain cell, which they call a “glutamatergic astrocyte,” could offer a way to treat Alzheimer’s, Parkinson’s, and other disorders of the nervous system.
“Its discovery opens up immense research prospects,” said study co-director Andrea Volterra.
The study: Neurotransmission starts with a neuron releasing a chemical called a neurotransmitter, so the first thing the researchers did in their study was look at whether astrocytes can release the main neurotransmitter used by neurons: glutamate.
By analyzing astrocytes taken from the brains of mice, they discovered that certain astrocytes in the brain’s hippocampus did include the “molecular machinery” needed to excrete glutamate. They found evidence of the same machinery when they looked at datasets of human glial cells.
Finally, to demonstrate that these hybrid cells are actually playing a role in brain signaling, the researchers suppressed their ability to secrete glutamate in the brains of mice. This caused the rodents to experience memory problems.
“Our next studies will explore the potential protective role of this type of cell against memory impairment in Alzheimer’s disease, as well as its role in other regions and pathologies than those explored here,” said Andrea Volterra, University of Lausanne.
But why? The researchers aren’t sure why the brain needs glutamatergic astrocytes when it already has neurons, but Volterra suspects the hybrid brain cells may help with the distribution of signals — a single astrocyte can be in contact with thousands of synapses.
“Often, we have neuronal information that needs to spread to larger ensembles, and neurons are not very good for the coordination of this,” researcher Ludovic Telley told New Scientist.
Looking ahead: More research is needed to see how the new brain cell functions in people, but the discovery that it plays a role in memory in mice suggests it might be a worthwhile target for Alzheimer’s disease treatments.
The researchers also found evidence during their study that the cell might play a role in brain circuits linked to seizures and voluntary movements, meaning it’s also a new lead in the hunt for better epilepsy and Parkinson’s treatments.
“Our next studies will explore the potential protective role of this type of cell against memory impairment in Alzheimer’s disease, as well as its role in other regions and pathologies than those explored here,” said Volterra.