Tiny, tough “water bears” may help bring new vaccines and medicines to sub-Saharan Africa
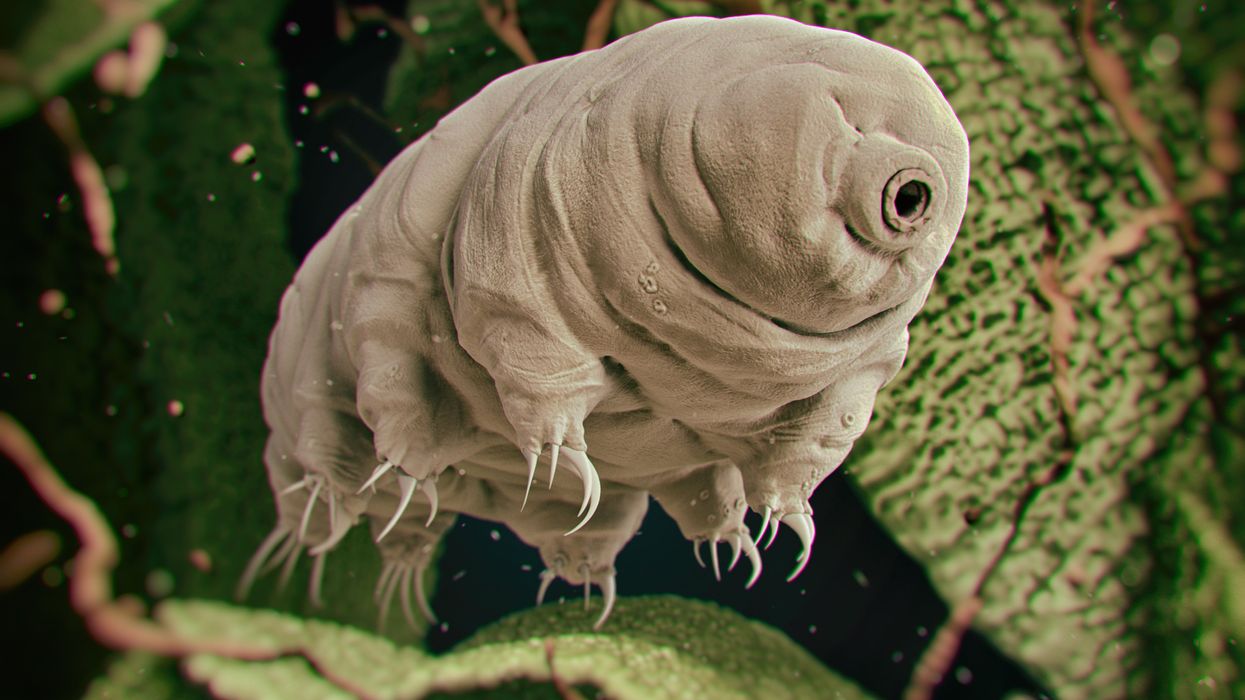
Tardigrades can completely dehydrate and later rehydrate themselves, a survival trick that scientists are harnessing to preserve medicines in hot temperatures.
Microscopic tardigrades, widely considered to be some of the toughest animals on earth, can survive for decades without oxygen or water and are thought to have lived through a crash-landing on the moon. Also known as water bears, they survive by fully dehydrating and later rehydrating themselves – a feat only a few animals can accomplish. Now scientists are harnessing tardigrades’ talents to make medicines that can be dried and stored at ambient temperatures and later rehydrated for use—instead of being kept refrigerated or frozen.
Many biologics—pharmaceutical products made by using living cells or synthesized from biological sources—require refrigeration, which isn’t always available in many remote locales or places with unreliable electricity. These products include mRNA and other vaccines, monoclonal antibodies and immuno-therapies for cancer, rheumatoid arthritis and other conditions. Cooling is also needed for medicines for blood clotting disorders like hemophilia and for trauma patients.
Formulating biologics to withstand drying and hot temperatures has been the holy grail for pharmaceutical researchers for decades. It’s a hard feat to manage. “Biologic pharmaceuticals are highly efficacious, but many are inherently unstable,” says Thomas Boothby, assistant professor of molecular biology at University of Wyoming. Therefore, during storage and shipping, they must be refrigerated at 2 to 8 degrees Celsius (35 to 46 degrees Fahrenheit). Some must be frozen, typically at -20 degrees Celsius, but sometimes as low -90 degrees Celsius as was the case with the Pfizer Covid vaccine.
For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
The costly cold chain
The logistics network that ensures those temperature requirements are met from production to administration is called the cold chain. This cold chain network is often unreliable or entirely lacking in remote, rural areas in developing nations that have malfunctioning electrical grids. “Almost all routine vaccines require a cold chain,” says Christopher Fox, senior vice president of formulations at the Access to Advanced Health Institute. But when the power goes out, so does refrigeration, putting refrigerated or frozen medical products at risk. Consequently, the mRNA vaccines developed for Covid-19 and other conditions, as well as more traditional vaccines for cholera, tetanus and other diseases, often can’t be delivered to the most remote parts of the world.
To understand the scope of the challenge, consider this: In the U.S., more than 984 million doses of Covid-19 vaccine have been distributed so far. Each one needed refrigeration that, even in the U.S., proved challenging. Now extrapolate to all vaccines and the entire world. For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
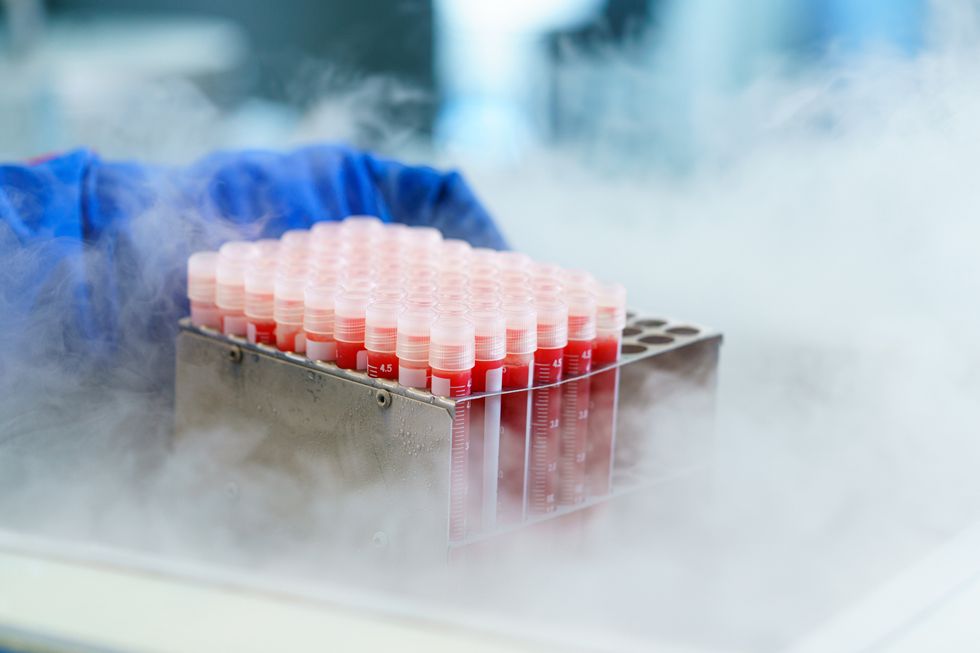
Globally, the cold chain packaging market is valued at over $15 billion and is expected to exceed $60 billion by 2033.
Adobe Stock
Freeze-drying, also called lyophilization, which is common for many vaccines, isn’t always an option. Many freeze-dried vaccines still need refrigeration, and even medicines approved for storage at ambient temperatures break down in the heat of sub-Saharan Africa. “Even in a freeze-dried state, biologics often will undergo partial rehydration and dehydration, which can be extremely damaging,” Boothby explains.
The cold chain is also very expensive to maintain. The global pharmaceutical cold chain packaging market is valued at more than $15 billion, and is expected to exceed $60 billion by 2033, according to a report by Future Market Insights. This cost is only expected to grow. According to the consulting company Accenture, the number of medicines that require the cold chain are expected to grow by 48 percent, compared to only 21 percent for non-cold-chain therapies.
Tardigrades to the rescue
Tardigrades are only about a millimeter long – with four legs and claws, and they lumber around like bears, thus their nickname – but could provide a big solution. “Tardigrades are unique in the animal kingdom, in that they’re able to survive a vast array of environmental insults,” says Boothby, the Wyoming professor. “They can be dried out, frozen, heated past the boiling point of water and irradiated at levels that are thousands of times more than you or I could survive.” So, his team is gradually unlocking tardigrades’ survival secrets and applying them to biologic pharmaceuticals to make them withstand both extreme heat and desiccation without losing efficacy.
Boothby’s team is focusing on blood clotting factor VIII, which, as the name implies, causes blood to clot. Currently, Boothby is concentrating on the so-called cytoplasmic abundant heat soluble (CAHS) protein family, which is found only in tardigrades, protecting them when they dry out. “We showed we can desiccate a biologic (blood clotting factor VIII, a key clotting component) in the presence of tardigrade proteins,” he says—without losing any of its effectiveness.
The researchers mixed the tardigrade protein with the blood clotting factor and then dried and rehydrated that substance six times without damaging the latter. This suggests that biologics protected with tardigrade proteins can withstand real-world fluctuations in humidity.
Furthermore, Boothby’s team found that when the blood clotting factor was dried and stabilized with tardigrade proteins, it retained its efficacy at temperatures as high as 95 degrees Celsius. That’s over 200 degrees Fahrenheit, much hotter than the 58 degrees Celsius that the World Meteorological Organization lists as the hottest recorded air temperature on earth. In contrast, without the protein, the blood clotting factor degraded significantly. The team published their findings in the journal Nature in March.
Although tardigrades rarely live more than 2.5 years, they have survived in a desiccated state for up to two decades, according to Animal Diversity Web. This suggests that tardigrades’ CAHS protein can protect biologic pharmaceuticals nearly indefinitely without refrigeration or freezing, which makes it significantly easier to deliver them in locations where refrigeration is unreliable or doesn’t exist.
The tricks of the tardigrades
Besides the CAHS proteins, tardigrades rely on a type of sugar called trehalose and some other protectants. So, rather than drying up, their cells solidify into rigid, glass-like structures. As that happens, viscosity between cells increases, thereby slowing their biological functions so much that they all but stop.
Now Boothby is combining CAHS D, one of the proteins in the CAHS family, with trehalose. He found that CAHS D and trehalose each protected proteins through repeated drying and rehydrating cycles. They also work synergistically, which means that together they might stabilize biologics under a variety of dry storage conditions.
“We’re finding the protective effect is not just additive but actually is synergistic,” he says. “We’re keen to see if something like that also holds true with different protein combinations.” If so, combinations could possibly protect against a variety of conditions.
Commercialization outlook
Before any stabilization technology for biologics can be commercialized, it first must be approved by the appropriate regulators. In the U.S., that’s the U.S. Food and Drug Administration. Developing a new formulation would require clinical testing and vast numbers of participants. So existing vaccines and biologics likely won’t be re-formulated for dry storage. “Many were developed decades ago,” says Fox. “They‘re not going to be reformulated into thermo-stable vaccines overnight,” if ever, he predicts.
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits.
Instead, this technology is most likely to be used for the new products and formulations that are just being created. New and improved vaccines will be the first to benefit. Good candidates include the plethora of mRNA vaccines, as well as biologic pharmaceuticals for neglected diseases that affect parts of the world where reliable cold chain is difficult to maintain, Boothby says. Some examples include new, more effective vaccines for malaria and for pathogenic Escherichia coli, which causes diarrhea.
Tallying up the benefits
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits. For instance, MenAfriVac, a meningitis vaccine (without tardigrade proteins) developed for sub-Saharan Africa, can be stored at up to 40 degrees Celsius for four days before administration. “If you have a few days where you don’t need to maintain the cold chain, it’s easier to transport vaccines to remote areas,” Fox says, where refrigeration does not exist or is not reliable.
Better health is an obvious benefit. MenAfriVac reduced suspected meningitis cases by 57 percent in the overall population and more than 99 percent among vaccinated individuals.
Lower healthcare costs are another benefit. One study done in Togo found that the cold chain-related costs increased the per dose vaccine price up to 11-fold. The ability to ship the vaccines using the usual cold chain, but transporting them at ambient temperatures for the final few days cut the cost in half.
There are environmental benefits, too, such as reducing fuel consumption and greenhouse gas emissions. Cold chain transports consume 20 percent more fuel than non-cold chain shipping, due to refrigeration equipment, according to the International Trade Administration.
A study by researchers at Johns Hopkins University compared the greenhouse gas emissions of the new, oral Vaxart COVID-19 vaccine (which doesn’t require refrigeration) with four intramuscular vaccines (which require refrigeration or freezing). While the Vaxart vaccine is still in clinical trials, the study found that “up to 82.25 million kilograms of CO2 could be averted by using oral vaccines in the U.S. alone.” That is akin to taking 17,700 vehicles out of service for one year.
Although tardigrades’ protective proteins won’t be a component of biologic pharmaceutics for several years, scientists are proving that this approach is viable. They are hopeful that a day will come when vaccines and biologics can be delivered anywhere in the world without needing refrigerators or freezers en route.
Podcast: Has the First 150-Year-Old Already Been Born
In today's podcast episode, Steven Austad explains why we should want to live a long time as long as that involves longer healthspans.
Steven Austad is a pioneer in the field of aging, with over 200 scientific papers and book chapters on pretty much every aspect of biological aging that you could think of. He’s also a strong believer in the potential for anti-aging therapies, and he puts his money where his mouth is. In 2001, he bet a billion dollars that the first person to reach 150-years-old had already been born. I had a chance to talk with Steven for today’s podcast and asked if he still thinks the bet was a good idea, since the oldest person so far (that we know of), Jeanne Calment, died back in 1997. A few days after our conversation, the oldest person in the world, Kane Tanaka, died at 119.
Steven is the Protective Life Endowed Chair in Health Aging Research, a Distinguished Professor and Chair of the Department of Biology at the University of Alabama Birmingham. He's also Senior Scientific Director of the American Federation for Aging Research, which is managing a groundbreaking longevity research trial that started this year. Steven is also a great science communicator with five books, including one that comes out later this year, Methuselah’s Zoo, and he publishes prolifically in national media outlets.
See the rest of his bio linked below in the show notes.
Listen to the Episode
Listen on Apple | Listen on Spotify | Listen on Stitcher | Listen on Amazon | Listen on Google

Steven Austad is featured in the latest episode of Making Sense of Science. He's a distinguished professor of biology at the University of Alabama Birmingham and has a new book due to be published in August, Methuselah's Zoo.
Photo by Steve Wood
Show notes:
2:36 - Steven explains why a particular opossum convinced him to dedicate his career to studying longevity.
6:48 - Steven's billion dollar bet that someone alive today will make it to 150-years-old.
9:15 - The most likely people to make it to 150 (Hint: not men).
10:38 - I ask Steven about Elon Musk’s comments this month that if people lived a really long time, “we’d be stuck with old ideas and society wouldn’t advance.” Steve isn’t so fond of that take.
13:34 - Why women are winning maybe the most important battle of sexes: staying alive. This is an area that Steven has led research on (see show notes).
18:20 - Why women, on average, actually have more morbidities earlier than men, even though they live longer.
23:10 - How the pandemic could affect sex differences in longevity.
24:55 - How often should people work out and get other physical activity to maximize longevity and health span?
29:09 - Steven gave me the latest update on the TAME trial on metformin, and how he and others longevity experts designed this groundbreaking research on longevity not in their offices, not on a zoom call, but in a castle in the Spanish countryside.
32:10 - Which anti-aging therapies are the most promising at this point for future research.
39:32 - The drug cocktail approach to address multiple hallmarks of aging.
41:00 - How to read health news like a scientist.
45:38 - Should we try a Manhattan project for aging?
48:47 - Can Jeff Bezos and Larry Ellison help us live to 150?
Show links:
Steven Austad's bio
Pre-order Steven's new book, Methuselah's Zoo - https://www.amazon.com/dp/B09M2QGRJR/ref=dp-kindle-redirect?_encoding=UTF8&btkr=1
Steven's journal article on Sex Differences in Lifespan - https://pubmed.ncbi.nlm.nih.gov/27304504/
Elon Musk's comments on super longevity "asphyxiating" society - https://www.cnbc.com/2022/04/11/elon-musk-on-avoid...
Steven's article on how to read news articles about health like a pro - https://www.nextavenue.org/how-to-read-health-news...
AFAR's research on Targeting Aging with Metformin (TAME) - https://www.afar.org/tame-trial
New therapy may improve stem cell transplants for blood cancers
Ivan Dimov, Jeroen Bekaert and Nate Fernhoff - pictured here - recognized the need for a more effective cell sorting technology to reduce the risk of Graft vs Host disease, which affects many cancer patients after receiving stem cell transplants.
In 2018, Robyn was diagnosed with myelofibrosis, a blood cancer causing chronic inflammation and scarring. As a research scientist by training, she knew she had limited options. A stem cell transplant is a terminally ill patient's best chance for survival against blood cancers, including leukaemia. It works by destroying a patient's cancer cells and replacing them with healthy cells from a donor.
However, there is a huge risk of Graft vs Host disease (GVHD), which affects around 30-40% of recipients. Patients receive billions of cells in a stem cell transplant but only a fraction are beneficial. The rest can attack healthy tissue leading to GVHD. It affects the skin, gut and lungs and can be truly debilitating.
Currently, steroids are used to try and prevent GVHD, but they have many side effects and are effective in only 50% of cases. “I spoke with my doctors and reached out to patients managing GVHD,” says Robyn, who prefers not to use her last name for privacy reasons. “My concerns really escalated for what I might face post-transplant.”
Then she heard about a new highly precise cell therapy developed by a company called Orca Bio, which gives patients more beneficial cells and fewer cells that cause GVHD. She decided to take part in their phase 2 trial.
How It Works
In stem cell transplants, patients receive immune cells and stem cells. The donor immune cells or T cells attack and kill malignant cells. This is the graft vs leukaemia effect (GVL). The stem cells generate new healthy cells.
Unfortunately, T cells can also cause GVHD, but a rare subset of T cells, called T regulatory cells, can actually prevent GVHD.
Orca’s cell sorting technology distinguishes T regulatory cells from stem cells and conventional T cells on a large scale. It’s this cell sorting technology which has enabled them to create their new cell therapy, called Orca T. It contains a precise combination of stem cells and immune cells with more T regulatory cells and fewer conventional T cells than in a typical stem cell transplant.
“Ivan Dimov’s idea was to spread out the cells, keep them stationary and then use laser scanning to sort the cells,” explains Nate Fernhoff, co-founder of Orca Bio. “The beauty here is that lasers don't care how quickly you move them.”
Over the past 40 years, scientists have been trying to create stem cell grafts that contain the beneficial cells whilst removing the cells that cause GVHD. What makes it even harder is that most transplant centers aren’t able to manipulate grafts to create a precise combination of cells.
Innovative Cell Sorting
Ivan Dimov, Jeroen Bekaert and Nate Fernhoff came up with the idea behind Orca as postdocs at Stanford, working with cell pioneer Irving Weissman. They recognised the need for a more effective cell sorting technology. In a small study at Stanford, Professor Robert Negrin had discovered a combination of T cells, T regulatory cells and stem cells which prevented GVHD but retained the beneficial graft vs leukaemia effect (GVL). However, manufacturing was problematic. Conventional cell sorting is extremely slow and specific. Negrin was only able to make seven highly precise products, for seven patients, in a year. Annual worldwide cases of blood cancer number over 1.2 million.
“We started Orca with this idea: how do we use manufacturing solutions to impact cell therapies,” co-founder Fernhoff reveals. In conventional cell sorting, cells move past a stationary laser which analyses each cell. But cells can only be moved so quickly. At a certain point they start to experience stress and break down. This makes it very difficult to sort the 100 billion cells from a donor in a stem cell transplant.
“Ivan Dimov’s idea was to spread out the cells, keep them stationary and then use laser scanning to sort the cells,” Fernhoff explains. “The beauty here is that lasers don't care how quickly you move them.” They developed this technology and called it Orca Sort. It enabled Orca to make up to six products per week in the first year of manufacturing.
Every product Orca makes is for one patient. The donor is uniquely matched to the patient. They have to carry out the cell sorting procedure each time. Everything also has to be done extremely quickly. They infuse fresh living cells from the donor's vein to the patient's within 72 hours.
“We’ve treated almost 200 patients in all the Orca trials, and you can't do that if you don't fix the manufacturing process,” Fernhoff says. “We're working on what we think is an incredibly promising drug, but it's all been enabled by figuring out how to make a high precision cell therapy at scale.”
Clinical Trials
Orca revealed the results of their phase 1b and phase 2 trials at the end of last year. In their phase 2 trial only 3% of the 29 patients treated with Orca T cell therapy developed chronic GVHD in the first year after treatment. Comparatively, 43% of the 95 patients given a conventional stem cell transplant in a contemporary Stanford trial developed chronic GVHD. Of the 109 patients tested in phase 1b and phase 2 trials, 74% using Orca T didn't relapse or develop any form of GVHD compared to 34% in the control trial.
“Until a randomised study is done, we can make no assumption about the relative efficacy of this approach," says Jeff Szer, professor of haematology at the Royal Melbourne Hospital. "But the holy grail of separating GVHD and GVL is still there and this is a step towards realising that dream.”
Stan Riddell, an immunology professor, at Fred Hutchinson Cancer Centre, believes Orca T is highly promising. “Orca has advanced cell selection processes with innovative methodology and can engineer grafts with greater precision to add cell subsets that may further contribute to beneficial outcomes,” he says. “Their results in phase 1 and phase 2 studies are very exciting and offer the potential of providing a new standard of care for stem cell transplant.”
However, though it is an “intriguing step,” there’s a need for further testing, according to Jeff Szer, a professor of haematology at the Peter MacCallum Cancer Centre at the Royal Melbourne Hospital.
“The numbers tested were tiny and comparing the outcomes to anything from a phase 1/2 setting is risky,” says Szer. “Until a randomised study is done, we can make no assumption about the relative efficacy of this approach. But the holy grail of separating GVHD and GVL is still there and this is a step towards realising that dream.”
The Future
The team is soon starting Phase 3 trials for Orca T. Its previous success has led them to develop Orca Q, a cell therapy for patients who can't find an exact donor match. Transplants for patients who are only a half-match or mismatched are not widely used because there is a greater risk of GVHD. Orca Q has the potential to control GVHD even more and improve access to transplants for many patients.
Fernhoff hopes they’ll be able to help people not just with blood cancers but also with other blood and immune disorders. If a patient has a debilitating disease which isn't life threatening, the risk of GVHD outweighs the potential benefits of a stem cell transplant. The Orca products could take away that risk.
Meanwhile, Robyn has no regrets about participating in the Phase 2 trial. “It was a serious decision to make but I'm forever grateful that I did,” she says. “I have resumed a quality of life aligned with how I felt pre-transplant. I have not had a single issue with GVHD.”
“I want to be able to get one of these products to every patient who could benefit from it,” Fernhoff says. “It's really exciting to think about how Orca's products could be applied to all sorts of autoimmune disorders.”

