Tiny, tough “water bears” may help bring new vaccines and medicines to sub-Saharan Africa
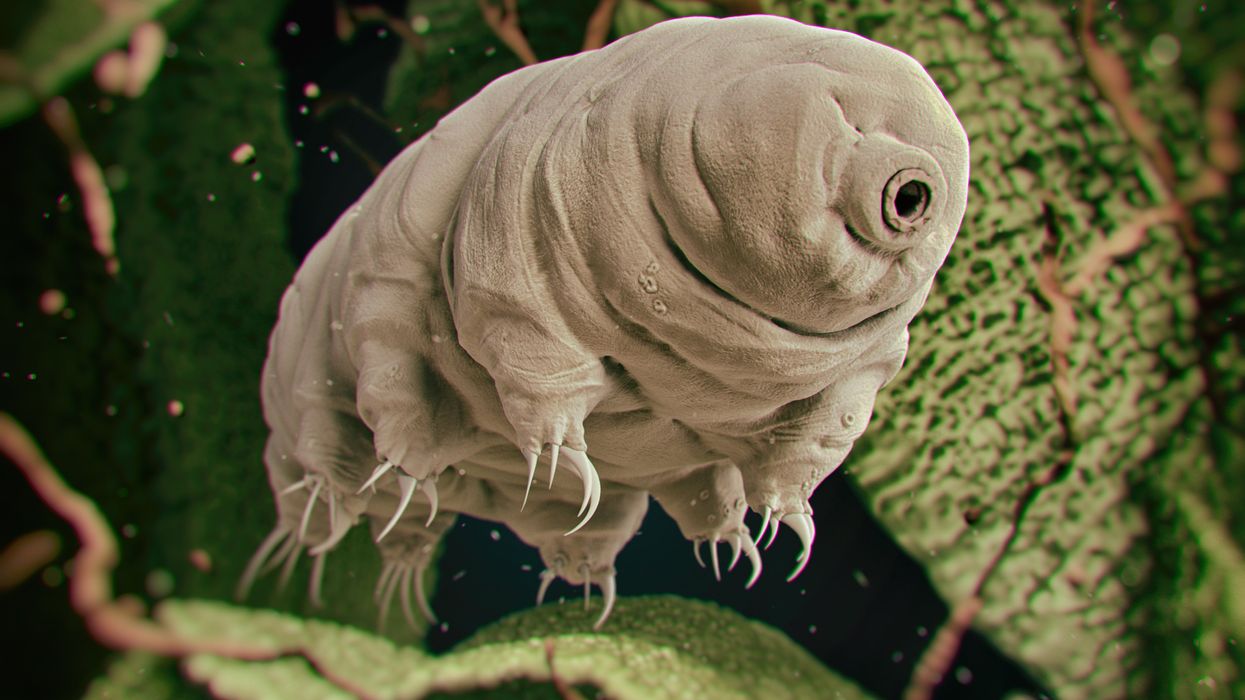
Tardigrades can completely dehydrate and later rehydrate themselves, a survival trick that scientists are harnessing to preserve medicines in hot temperatures.
Microscopic tardigrades, widely considered to be some of the toughest animals on earth, can survive for decades without oxygen or water and are thought to have lived through a crash-landing on the moon. Also known as water bears, they survive by fully dehydrating and later rehydrating themselves – a feat only a few animals can accomplish. Now scientists are harnessing tardigrades’ talents to make medicines that can be dried and stored at ambient temperatures and later rehydrated for use—instead of being kept refrigerated or frozen.
Many biologics—pharmaceutical products made by using living cells or synthesized from biological sources—require refrigeration, which isn’t always available in many remote locales or places with unreliable electricity. These products include mRNA and other vaccines, monoclonal antibodies and immuno-therapies for cancer, rheumatoid arthritis and other conditions. Cooling is also needed for medicines for blood clotting disorders like hemophilia and for trauma patients.
Formulating biologics to withstand drying and hot temperatures has been the holy grail for pharmaceutical researchers for decades. It’s a hard feat to manage. “Biologic pharmaceuticals are highly efficacious, but many are inherently unstable,” says Thomas Boothby, assistant professor of molecular biology at University of Wyoming. Therefore, during storage and shipping, they must be refrigerated at 2 to 8 degrees Celsius (35 to 46 degrees Fahrenheit). Some must be frozen, typically at -20 degrees Celsius, but sometimes as low -90 degrees Celsius as was the case with the Pfizer Covid vaccine.
For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
The costly cold chain
The logistics network that ensures those temperature requirements are met from production to administration is called the cold chain. This cold chain network is often unreliable or entirely lacking in remote, rural areas in developing nations that have malfunctioning electrical grids. “Almost all routine vaccines require a cold chain,” says Christopher Fox, senior vice president of formulations at the Access to Advanced Health Institute. But when the power goes out, so does refrigeration, putting refrigerated or frozen medical products at risk. Consequently, the mRNA vaccines developed for Covid-19 and other conditions, as well as more traditional vaccines for cholera, tetanus and other diseases, often can’t be delivered to the most remote parts of the world.
To understand the scope of the challenge, consider this: In the U.S., more than 984 million doses of Covid-19 vaccine have been distributed so far. Each one needed refrigeration that, even in the U.S., proved challenging. Now extrapolate to all vaccines and the entire world. For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
Globally, the cold chain packaging market is valued at over $15 billion and is expected to exceed $60 billion by 2033.
Adobe Stock
Freeze-drying, also called lyophilization, which is common for many vaccines, isn’t always an option. Many freeze-dried vaccines still need refrigeration, and even medicines approved for storage at ambient temperatures break down in the heat of sub-Saharan Africa. “Even in a freeze-dried state, biologics often will undergo partial rehydration and dehydration, which can be extremely damaging,” Boothby explains.
The cold chain is also very expensive to maintain. The global pharmaceutical cold chain packaging market is valued at more than $15 billion, and is expected to exceed $60 billion by 2033, according to a report by Future Market Insights. This cost is only expected to grow. According to the consulting company Accenture, the number of medicines that require the cold chain are expected to grow by 48 percent, compared to only 21 percent for non-cold-chain therapies.
Tardigrades to the rescue
Tardigrades are only about a millimeter long – with four legs and claws, and they lumber around like bears, thus their nickname – but could provide a big solution. “Tardigrades are unique in the animal kingdom, in that they’re able to survive a vast array of environmental insults,” says Boothby, the Wyoming professor. “They can be dried out, frozen, heated past the boiling point of water and irradiated at levels that are thousands of times more than you or I could survive.” So, his team is gradually unlocking tardigrades’ survival secrets and applying them to biologic pharmaceuticals to make them withstand both extreme heat and desiccation without losing efficacy.
Boothby’s team is focusing on blood clotting factor VIII, which, as the name implies, causes blood to clot. Currently, Boothby is concentrating on the so-called cytoplasmic abundant heat soluble (CAHS) protein family, which is found only in tardigrades, protecting them when they dry out. “We showed we can desiccate a biologic (blood clotting factor VIII, a key clotting component) in the presence of tardigrade proteins,” he says—without losing any of its effectiveness.
The researchers mixed the tardigrade protein with the blood clotting factor and then dried and rehydrated that substance six times without damaging the latter. This suggests that biologics protected with tardigrade proteins can withstand real-world fluctuations in humidity.
Furthermore, Boothby’s team found that when the blood clotting factor was dried and stabilized with tardigrade proteins, it retained its efficacy at temperatures as high as 95 degrees Celsius. That’s over 200 degrees Fahrenheit, much hotter than the 58 degrees Celsius that the World Meteorological Organization lists as the hottest recorded air temperature on earth. In contrast, without the protein, the blood clotting factor degraded significantly. The team published their findings in the journal Nature in March.
Although tardigrades rarely live more than 2.5 years, they have survived in a desiccated state for up to two decades, according to Animal Diversity Web. This suggests that tardigrades’ CAHS protein can protect biologic pharmaceuticals nearly indefinitely without refrigeration or freezing, which makes it significantly easier to deliver them in locations where refrigeration is unreliable or doesn’t exist.
The tricks of the tardigrades
Besides the CAHS proteins, tardigrades rely on a type of sugar called trehalose and some other protectants. So, rather than drying up, their cells solidify into rigid, glass-like structures. As that happens, viscosity between cells increases, thereby slowing their biological functions so much that they all but stop.
Now Boothby is combining CAHS D, one of the proteins in the CAHS family, with trehalose. He found that CAHS D and trehalose each protected proteins through repeated drying and rehydrating cycles. They also work synergistically, which means that together they might stabilize biologics under a variety of dry storage conditions.
“We’re finding the protective effect is not just additive but actually is synergistic,” he says. “We’re keen to see if something like that also holds true with different protein combinations.” If so, combinations could possibly protect against a variety of conditions.
Commercialization outlook
Before any stabilization technology for biologics can be commercialized, it first must be approved by the appropriate regulators. In the U.S., that’s the U.S. Food and Drug Administration. Developing a new formulation would require clinical testing and vast numbers of participants. So existing vaccines and biologics likely won’t be re-formulated for dry storage. “Many were developed decades ago,” says Fox. “They‘re not going to be reformulated into thermo-stable vaccines overnight,” if ever, he predicts.
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits.
Instead, this technology is most likely to be used for the new products and formulations that are just being created. New and improved vaccines will be the first to benefit. Good candidates include the plethora of mRNA vaccines, as well as biologic pharmaceuticals for neglected diseases that affect parts of the world where reliable cold chain is difficult to maintain, Boothby says. Some examples include new, more effective vaccines for malaria and for pathogenic Escherichia coli, which causes diarrhea.
Tallying up the benefits
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits. For instance, MenAfriVac, a meningitis vaccine (without tardigrade proteins) developed for sub-Saharan Africa, can be stored at up to 40 degrees Celsius for four days before administration. “If you have a few days where you don’t need to maintain the cold chain, it’s easier to transport vaccines to remote areas,” Fox says, where refrigeration does not exist or is not reliable.
Better health is an obvious benefit. MenAfriVac reduced suspected meningitis cases by 57 percent in the overall population and more than 99 percent among vaccinated individuals.
Lower healthcare costs are another benefit. One study done in Togo found that the cold chain-related costs increased the per dose vaccine price up to 11-fold. The ability to ship the vaccines using the usual cold chain, but transporting them at ambient temperatures for the final few days cut the cost in half.
There are environmental benefits, too, such as reducing fuel consumption and greenhouse gas emissions. Cold chain transports consume 20 percent more fuel than non-cold chain shipping, due to refrigeration equipment, according to the International Trade Administration.
A study by researchers at Johns Hopkins University compared the greenhouse gas emissions of the new, oral Vaxart COVID-19 vaccine (which doesn’t require refrigeration) with four intramuscular vaccines (which require refrigeration or freezing). While the Vaxart vaccine is still in clinical trials, the study found that “up to 82.25 million kilograms of CO2 could be averted by using oral vaccines in the U.S. alone.” That is akin to taking 17,700 vehicles out of service for one year.
Although tardigrades’ protective proteins won’t be a component of biologic pharmaceutics for several years, scientists are proving that this approach is viable. They are hopeful that a day will come when vaccines and biologics can be delivered anywhere in the world without needing refrigerators or freezers en route.
Food Poisoning Sickens Millions a Year. Now, a Surprising Weapon Is Helping Protect Against Contamination.
Phages, which are harmless viruses that destroy specific bacteria, are becoming useful tools to protect our food supply.
Every year, one in seven people in America comes down with a foodborne illness, typically caused by a bacterial pathogen, including E.Coli, listeria, salmonella, or campylobacter. That adds up to 48 million people, of which 120,000 are hospitalized and 3000 die, according to the Centers for Disease Control. And the variety of foods that can be contaminated with bacterial pathogens is growing too. In the 20th century, E.Coli and listeria lurked primarily within meat. Now they find their way into lettuce, spinach, and other leafy greens, causing periodic consumer scares and product recalls. Onions are the most recent suspected culprit of a nationwide salmonella outbreak.
Some of these incidents are almost inevitable because of how Mother Nature works, explains Divya Jaroni, associate professor of animal and food sciences at Oklahoma State University. These common foodborne pathogens come from the cattle's intestines when the animals shed them in their manure—and then they get washed into rivers and lakes, especially in heavy rains. When this water is later used to irrigate produce farms, the bugs end up on salad greens. Plus, many small farms do both—herd cattle and grow produce.
"Unfortunately for us, these pathogens are part of the microflora of the cows' intestinal tract," Jaroni says. "Some farmers may have an acre or two of cattle pastures, and an acre of a produce farm nearby, so it's easy for this water to contaminate the crops."
Food producers and packagers fight bacteria by potent chemicals, with chlorine being the go-to disinfectant. Cattle carcasses, for example, are typically washed by chlorine solutions as the animals' intestines are removed. Leafy greens are bathed in water with added chlorine solutions. However, because the same "bath" can be used for multiple veggie batches and chlorine evaporates over time, the later rounds may not kill all of the bacteria, sparing some. The natural and organic producers avoid chlorine, substituting it with lactic acid, a more holistic sanitizer, but even with all these efforts, some pathogens survive, sickening consumers and causing food recalls. As we farm more animals and grow more produce, while also striving to use fewer chemicals and more organic growing methods, it will be harder to control bacteria's spread.
"It took us a long time to convince the FDA phages were safe and efficient alternatives. But we had worked with them to gather all the data they needed, and the FDA was very supportive in the end."
Luckily, bacteria have their own killers. Called bacteriophages, or phages for short, they are viruses that prey on bacteria only. Under the electron microscope, they look like fantasy spaceships, with oblong bodies, spider-like legs and long tails. Much smaller than a bacterium, phages pierce the microbes' cells with their tails, sneak in and begin multiplying inside, eventually bursting the microbes open—and then proceed to infect more of them. The best part is that these phages are harmless to humans. Moreover, recent research finds that millions of phages dwell on us and in us—in our nose, throat, skin and gut, protecting us from bacterial infections as part of our healthy microbiome. A recent study suggested that we absorb about 30 billion phages into our bodies on a daily basis. Now, ingeniously, they are starting to be deployed as anti-microbial agents in the food industry.
A Maryland-based phage research company called Intralytix is doing just that. Founded by Alexander Sulakvelidze, a microbiologist and epidemiologist who came to the United States from Tbilisi, the capital of Georgia, Intralytix makes and sells five different FDA-approved phage cocktails that work against some of the most notorious food pathogens: ListShield for Listeria, SalmoFresh for Salmonella, ShigaShield for Shigella, another foodborne bug, and EcoShield for E.coli, including the infamous strain that caused the Jack in the Box outbreak in 1993 that killed four children and sickened 732 people across four states. Earlier this month, the FDA granted its approval to yet another Intralytix phage for managing Campylobacter contamination, named CampyShield. "We call it safety by nature," Sulakvelidze says.

Intralytix grows phages inside massive 1500-liter fermenters, feeding them bacterial "fodder."
Photo credit: Living Radiant Photography
Phage preparations are relatively straightforward to make. In nature, phages thrive in any body of water where bacteria live too, including rivers, lakes and bays. "I can dip a bucket into the Chesapeake Bay, and it will be full of all kinds of phages," Sulakvelidze says. "Sewage is another great place to look for specific phages of interest, because it's teeming with all sorts of bacteria—and therefore the viruses that prey on them." In lab settings, Intralytix grows phages inside massive 1500-liter fermenters, feeding them bacterial "fodder." Once phages multiply enough, they are harvested, dispensed into containers and shipped to food producers who have adopted this disinfecting practice into their preparation process. Typically, it's done by computer-controlled sprayer systems that disperse mist-like phage preparations onto the food.
Unlike chemicals like chlorine or antibiotics, which kill a wide spectrum of bacteria, phages are more specialized, each feeding on specific microbial species. A phage that targets salmonella will not prey on listeria and vice versa. So food producers may sometimes use a combo of different phage preparations. Intralytix is continuously researching and testing new phages. With a contract from the National Institutes of Health, Intralytix is expanding its automated high-throughput robot that tests which phages work best against which bacteria, speeding up the development of the new phage cocktails.
Phages have other "talents." In her recent study, Jaroni found that phages have the ability to destroy bacterial biofilms—colonies of microorganisms that tend to grow on surfaces of the food processing equipment, surrounding themselves with protective coating that even very harsh chemicals can't crack. "Phages are very clever," Jaroni says. "They produce enzymes that target the biofilms, and once they break through, they can reach the bacteria."
Convincing the FDA that phages were safe to use on food products was no easy feat, Sulakvelidze says. In his home country of Georgia, phages have been used as antimicrobial remedies for over a century, but the FDA was leery of using viruses as food safety agents. "It took us a long time to convince the FDA phages were safe and efficient alternatives," Sulakvelidze says. "But we had worked with them to gather all the data they needed, and the FDA was very supportive in the end." The agency had granted Intralytix its first approval in 2006, and over the past 10 years, the company's sales increased by over 15-fold. "We currently sell to about 40 companies and are in discussions with several other large food producers," Sulakvelidze says. One indicator that the industry now understands and appreciates the science of phages was that his company was ranked as Top Food Safety Provider in 2021 by Food and Beverage Technology Review, he adds. Notably, phage sprays are kosher, halal and organic-certified.
Intralytix's phage cocktails to safeguard food from bacteria are approved for consumers in addition to food producers, but currently the company sells to food producers only. Selling retail requires different packaging like easy-to-use spray bottles and different marketing that would inform people about phages' antimicrobial qualities. But ultimately, giving people the ability to remove pathogens from their food with probiotic phage sprays is the goal, Sulakvelidze says.
It's not the company's only goal. Now Intralytix is going a step further, investigating phages' probiotic and therapeutic abilities. Because phages are highly specialized in the bacteria they target, they can be used to treat infections caused by specific pathogens while leaving the beneficial species of our microbiome intact. In an ongoing clinical trial with Mount Sinai, Intralytix is now investigating a potential phage treatment against a certain type of E. coli for patients with Crohn's disease, and is about to start another clinical trial for treating bacterial dysentery.
"Now that we have proved that phages are safe and effective against foodborne bacteria," Sulakvelidze says, "we are going to demonstrate their potential in therapeutic applications."
Lina Zeldovich has written about science, medicine and technology for Popular Science, Smithsonian, National Geographic, Scientific American, Reader’s Digest, the New York Times and other major national and international publications. A Columbia J-School alumna, she has won several awards for her stories, including the ASJA Crisis Coverage Award for Covid reporting, and has been a contributing editor at Nautilus Magazine. In 2021, Zeldovich released her first book, The Other Dark Matter, published by the University of Chicago Press, about the science and business of turning waste into wealth and health. You can find her on http://linazeldovich.com/ and @linazeldovich.
Your Future Smartphone May Detect Problems in Your Water
Biosensors on a touchscreen are showing promise for detecting arsenic and lead in water.
In 2014, the city of Flint, Michigan switched the residents' water supply to the Flint river, citing cheaper costs. However, due to improper filtering, lead contaminated this water, and according to the Associated Press, many of the city's residents soon reported health issues like hair loss and rashes. In 2015, a report found that children there had high levels of lead in their blood. The National Resource Defense Council recently discovered there could still be as many as twelve million lead pipes carrying water to homes across the U.S.
What if Flint residents and others in afflicted areas could simply flick water onto their phone screens and an app would tell them if they were about to drink contaminated water? This is what researchers at the University of Cambridge are working on to prevent catastrophes like what occurred in Flint, and to prepare for an uncertain future of scarcer resources.
Underneath the tough glass of our phone screen lies a transparent layer of electrodes. Because our bodies hold an electric charge, when our finger touches the screen, it disrupts the electric field created among the electrodes. This is how the screen can sense where a touch occurs. Cambridge scientists used this same idea to explore whether the screen could detect charges in water, too. Metals like arsenic and lead can appear in water in the form of ions, which are charged particles. When the ionic solution is placed on the screen's surface, the electrodes sense that charge like how they sense our finger.
Imagine a new generation of smartphones with a designated area of the screen responsible for detecting contamination—this is one of the possible futures the researchers propose.
The experiment measured charges in various electrolyte solutions on a touchscreen. The researchers found that a thin polymer layer between the electrodes and the sample solution helped pick up the charges.
"How can we get really close to the touch electrodes, and be better than a phone screen?" Horstmann, the lead scientist on the study, asked himself while designing the protective coating. "We found that when we put electrolytes directly on the electrodes, they were too close, even short-circuiting," he said. When they placed the polymer layer on top the electrodes, however, this short-circuiting did not occur. Horstmann speaks of the polymer layer as one of the key findings of the paper, as it allowed for optimum conductivity. The coating they designed was much thinner than what you'd see with a typical smartphone touchscreen, but because it's already so similar, he feels optimistic about the technology's practical applications in the real world.
While the Cambridge scientists were using touchscreens to measure water contamination, Dr. Baojun Wang, a synthetic biologist at the University of Edinburgh, along with his team, created a way to measure arsenic contamination in Bangladesh groundwater samples using what is called a cell-based biosensor. These biosensors use cornerstones of cellular activity like transcription and promoter sequences to detect the presence of metal ions in water. A promoter can be thought of as a "flag" that tells certain molecules where to begin copying genetic code. By hijacking this aspect of the cell's machinery and increasing the cell's sensing and signal processing ability, they were able to amplify the signal to detect tiny amounts of arsenic in the groundwater samples. All this was conducted in a 384-well plate, each well smaller than a pencil eraser.
They placed arsenic sensors with different sensitivities across part of the plate so it resembled a volume bar of increasing levels of arsenic, similar to diagnostics on a Fitbit or glucose monitor. The whole device is about the size of an iPhone, and can be scaled down to a much smaller size.
Dr. Wang says cell-based biosensors are bringing sensing technology closer to field applications, because their machinery uses inherent cellular activity. This makes them ideal for low-resource communities, and he expects his device to be affordable, portable, and easily stored for widespread use in households.
"It hasn't worked on actual phones yet, but I don't see any reason why it can't be an app," says Horstmann of their technology. Imagine a new generation of smartphones with a designated area of the screen responsible for detecting contamination—this is one of the possible futures the researchers propose. But industry collaborations will be crucial to making their advancements practical. The scientists anticipate that without collaborative efforts from the business sector, the public might have to wait ten years until this becomes something all our smartphones are capable of—but with the right partners, "it could go really quickly," says Dr. Elizabeth Hall, one of the authors on the touchscreen water contamination study.
"That's where the science ends and the business begins," Dr. Hall says. "There is a lot of interest coming through as a result of this paper. I think the people who make the investments and decisions are seeing that there might be something useful here."
As for Flint, according to The Detroit News, the city has entered the final stages in removing lead pipe infrastructure. It's difficult to imagine how many residents might fare better today if they'd had the technology that scientists are now creating.
Of all its tragedy, COVID-19 has increased demand for at-home testing methods, which has carried over to non-COVID-19-related devices. Various testing efforts are now in the public eye.
"I like that the public is watching these directions," says Horstmann. "I think there's a long way to go still, but it's exciting."

