Tiny, tough “water bears” may help bring new vaccines and medicines to sub-Saharan Africa
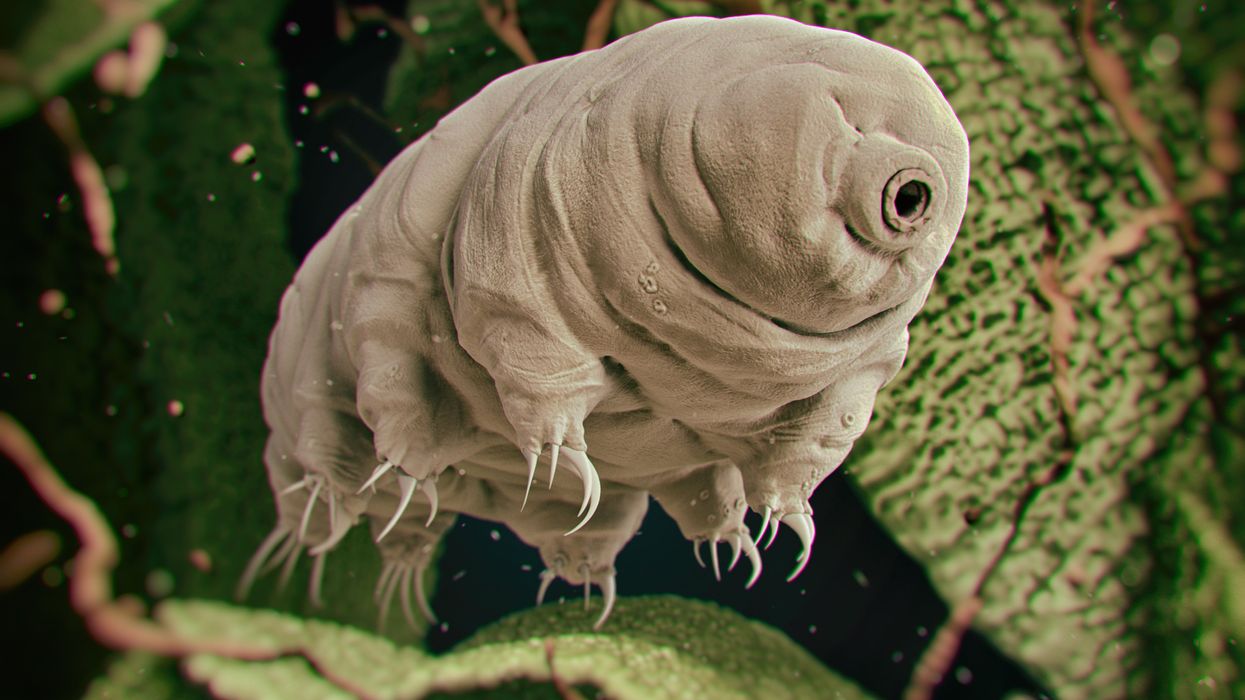
Tardigrades can completely dehydrate and later rehydrate themselves, a survival trick that scientists are harnessing to preserve medicines in hot temperatures.
Microscopic tardigrades, widely considered to be some of the toughest animals on earth, can survive for decades without oxygen or water and are thought to have lived through a crash-landing on the moon. Also known as water bears, they survive by fully dehydrating and later rehydrating themselves – a feat only a few animals can accomplish. Now scientists are harnessing tardigrades’ talents to make medicines that can be dried and stored at ambient temperatures and later rehydrated for use—instead of being kept refrigerated or frozen.
Many biologics—pharmaceutical products made by using living cells or synthesized from biological sources—require refrigeration, which isn’t always available in many remote locales or places with unreliable electricity. These products include mRNA and other vaccines, monoclonal antibodies and immuno-therapies for cancer, rheumatoid arthritis and other conditions. Cooling is also needed for medicines for blood clotting disorders like hemophilia and for trauma patients.
Formulating biologics to withstand drying and hot temperatures has been the holy grail for pharmaceutical researchers for decades. It’s a hard feat to manage. “Biologic pharmaceuticals are highly efficacious, but many are inherently unstable,” says Thomas Boothby, assistant professor of molecular biology at University of Wyoming. Therefore, during storage and shipping, they must be refrigerated at 2 to 8 degrees Celsius (35 to 46 degrees Fahrenheit). Some must be frozen, typically at -20 degrees Celsius, but sometimes as low -90 degrees Celsius as was the case with the Pfizer Covid vaccine.
For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
The costly cold chain
The logistics network that ensures those temperature requirements are met from production to administration is called the cold chain. This cold chain network is often unreliable or entirely lacking in remote, rural areas in developing nations that have malfunctioning electrical grids. “Almost all routine vaccines require a cold chain,” says Christopher Fox, senior vice president of formulations at the Access to Advanced Health Institute. But when the power goes out, so does refrigeration, putting refrigerated or frozen medical products at risk. Consequently, the mRNA vaccines developed for Covid-19 and other conditions, as well as more traditional vaccines for cholera, tetanus and other diseases, often can’t be delivered to the most remote parts of the world.
To understand the scope of the challenge, consider this: In the U.S., more than 984 million doses of Covid-19 vaccine have been distributed so far. Each one needed refrigeration that, even in the U.S., proved challenging. Now extrapolate to all vaccines and the entire world. For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
Globally, the cold chain packaging market is valued at over $15 billion and is expected to exceed $60 billion by 2033.
Adobe Stock
Freeze-drying, also called lyophilization, which is common for many vaccines, isn’t always an option. Many freeze-dried vaccines still need refrigeration, and even medicines approved for storage at ambient temperatures break down in the heat of sub-Saharan Africa. “Even in a freeze-dried state, biologics often will undergo partial rehydration and dehydration, which can be extremely damaging,” Boothby explains.
The cold chain is also very expensive to maintain. The global pharmaceutical cold chain packaging market is valued at more than $15 billion, and is expected to exceed $60 billion by 2033, according to a report by Future Market Insights. This cost is only expected to grow. According to the consulting company Accenture, the number of medicines that require the cold chain are expected to grow by 48 percent, compared to only 21 percent for non-cold-chain therapies.
Tardigrades to the rescue
Tardigrades are only about a millimeter long – with four legs and claws, and they lumber around like bears, thus their nickname – but could provide a big solution. “Tardigrades are unique in the animal kingdom, in that they’re able to survive a vast array of environmental insults,” says Boothby, the Wyoming professor. “They can be dried out, frozen, heated past the boiling point of water and irradiated at levels that are thousands of times more than you or I could survive.” So, his team is gradually unlocking tardigrades’ survival secrets and applying them to biologic pharmaceuticals to make them withstand both extreme heat and desiccation without losing efficacy.
Boothby’s team is focusing on blood clotting factor VIII, which, as the name implies, causes blood to clot. Currently, Boothby is concentrating on the so-called cytoplasmic abundant heat soluble (CAHS) protein family, which is found only in tardigrades, protecting them when they dry out. “We showed we can desiccate a biologic (blood clotting factor VIII, a key clotting component) in the presence of tardigrade proteins,” he says—without losing any of its effectiveness.
The researchers mixed the tardigrade protein with the blood clotting factor and then dried and rehydrated that substance six times without damaging the latter. This suggests that biologics protected with tardigrade proteins can withstand real-world fluctuations in humidity.
Furthermore, Boothby’s team found that when the blood clotting factor was dried and stabilized with tardigrade proteins, it retained its efficacy at temperatures as high as 95 degrees Celsius. That’s over 200 degrees Fahrenheit, much hotter than the 58 degrees Celsius that the World Meteorological Organization lists as the hottest recorded air temperature on earth. In contrast, without the protein, the blood clotting factor degraded significantly. The team published their findings in the journal Nature in March.
Although tardigrades rarely live more than 2.5 years, they have survived in a desiccated state for up to two decades, according to Animal Diversity Web. This suggests that tardigrades’ CAHS protein can protect biologic pharmaceuticals nearly indefinitely without refrigeration or freezing, which makes it significantly easier to deliver them in locations where refrigeration is unreliable or doesn’t exist.
The tricks of the tardigrades
Besides the CAHS proteins, tardigrades rely on a type of sugar called trehalose and some other protectants. So, rather than drying up, their cells solidify into rigid, glass-like structures. As that happens, viscosity between cells increases, thereby slowing their biological functions so much that they all but stop.
Now Boothby is combining CAHS D, one of the proteins in the CAHS family, with trehalose. He found that CAHS D and trehalose each protected proteins through repeated drying and rehydrating cycles. They also work synergistically, which means that together they might stabilize biologics under a variety of dry storage conditions.
“We’re finding the protective effect is not just additive but actually is synergistic,” he says. “We’re keen to see if something like that also holds true with different protein combinations.” If so, combinations could possibly protect against a variety of conditions.
Commercialization outlook
Before any stabilization technology for biologics can be commercialized, it first must be approved by the appropriate regulators. In the U.S., that’s the U.S. Food and Drug Administration. Developing a new formulation would require clinical testing and vast numbers of participants. So existing vaccines and biologics likely won’t be re-formulated for dry storage. “Many were developed decades ago,” says Fox. “They‘re not going to be reformulated into thermo-stable vaccines overnight,” if ever, he predicts.
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits.
Instead, this technology is most likely to be used for the new products and formulations that are just being created. New and improved vaccines will be the first to benefit. Good candidates include the plethora of mRNA vaccines, as well as biologic pharmaceuticals for neglected diseases that affect parts of the world where reliable cold chain is difficult to maintain, Boothby says. Some examples include new, more effective vaccines for malaria and for pathogenic Escherichia coli, which causes diarrhea.
Tallying up the benefits
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits. For instance, MenAfriVac, a meningitis vaccine (without tardigrade proteins) developed for sub-Saharan Africa, can be stored at up to 40 degrees Celsius for four days before administration. “If you have a few days where you don’t need to maintain the cold chain, it’s easier to transport vaccines to remote areas,” Fox says, where refrigeration does not exist or is not reliable.
Better health is an obvious benefit. MenAfriVac reduced suspected meningitis cases by 57 percent in the overall population and more than 99 percent among vaccinated individuals.
Lower healthcare costs are another benefit. One study done in Togo found that the cold chain-related costs increased the per dose vaccine price up to 11-fold. The ability to ship the vaccines using the usual cold chain, but transporting them at ambient temperatures for the final few days cut the cost in half.
There are environmental benefits, too, such as reducing fuel consumption and greenhouse gas emissions. Cold chain transports consume 20 percent more fuel than non-cold chain shipping, due to refrigeration equipment, according to the International Trade Administration.
A study by researchers at Johns Hopkins University compared the greenhouse gas emissions of the new, oral Vaxart COVID-19 vaccine (which doesn’t require refrigeration) with four intramuscular vaccines (which require refrigeration or freezing). While the Vaxart vaccine is still in clinical trials, the study found that “up to 82.25 million kilograms of CO2 could be averted by using oral vaccines in the U.S. alone.” That is akin to taking 17,700 vehicles out of service for one year.
Although tardigrades’ protective proteins won’t be a component of biologic pharmaceutics for several years, scientists are proving that this approach is viable. They are hopeful that a day will come when vaccines and biologics can be delivered anywhere in the world without needing refrigerators or freezers en route.
Donna Shirley pictured at her home in Tulsa, with a model of the Sojourner rover she was in charge of that explored Mars.
When NASA's Perseverance rover landed successfully on Mars on February 18, 2021, calling it "one giant leap for mankind" – as Neil Armstrong said when he set foot on the moon in 1969 – would have been inaccurate. This year actually marked the fifth time the U.S. space agency has put a remote-controlled robotic exploration vehicle on the Red Planet. And it was a female engineer named Donna Shirley who broke new ground for women in science as the manager of both the Mars Exploration Program and the 30-person team that built Sojourner, the first rover to land on Mars on July 4, 1997.
For Shirley, the Mars Pathfinder mission was the climax of her 32-year career at NASA's Jet Propulsion Laboratory (JPL) in Pasadena, California. The Oklahoma-born scientist, who earned her Master's degree in aerospace engineering from the University of Southern California, saw her profile skyrocket with media appearances from CNN to the New York Times, and her autobiography Managing Martians came out in 1998. Now 79 and living in a Tulsa retirement community, she still embraces her status as a female pioneer.
"Periodically, I'll hear somebody say they got into the space program because of me, and that makes me feel really good," Shirley told Leaps.org. "I look at the mission control area, and there are a lot of women in there. I'm quite pleased I was able to break the glass ceiling."
Her $25-million, 25-pound microrover – powered by solar energy and designed to get rock samples and test soil chemistry for evidence of life – was named after Sojourner Truth, a 19th-century Black abolitionist and women's rights activist. Unlike Mars Pathfinder, Shirley didn't have to travel more than 131 million miles to reach her goal, but her path to scientific fame as a woman sometimes resembled an asteroid field.
 The Sojourner Rover in 1997 on Mars.NASA/JPL
The Sojourner Rover in 1997 on Mars.NASA/JPLAs a high-IQ tomboy growing up in Wynnewood, Oklahoma (pop. 2,300), Shirley yearned to escape. She decided to become an engineer at age 10 and took flying lessons at 15. Her extraterrestrial aspirations were fueled by Ray Bradbury's The Martian Chronicles and Arthur C. Clarke's The Sands of Mars. Yet when she entered the University of Oklahoma (OU) in 1958, her freshman academic advisor initially told her: "Girls can't be engineers." She ignored him.
Years later, Shirley would combat such archaic thinking, succeeding at JPL with her creative, collaborative management style. "If you look at the literature, you'll find that teams that are either led by or heavily involved with women do better than strictly male teams," she noted.
However, her career trajectory stalled at OU. Burned out by her course load and distracted by a broken engagement to marry a fellow student, she switched her major to professional writing. After graduation, she applied her aeronautical background as a McDonnell Aircraft technical writer, but her boss, she says, harassed her and she faced gender-based hostility from male co-workers.
Returning to OU, Shirley finished off her engineering degree and became a JPL aerodynamist in 1966 after answering an ad in the St. Louis Post-Dispatch. At first, she was the only female engineer among the research center's 2,000-odd engineers. She wore many hats, from designing planetary atmospheric entry vehicles to picking the launch date of November 4, 1973 for Mariner 10's mission to Venus and Mercury.
By the mid-1980's, she was managing teams that focused on robotics and Mars, delivering creative solutions when NASA budget cuts loomed. In 1989, the same year the Sojourner microrover concept was born, President George H.W. Bush announced his Space Exploration Initiative, including plans for a human mission to Mars by 2019.
That target, of course, wasn't attained, despite huge advances in technology and our understanding of the Martian environment. Today, Shirley believes humans could land on Mars by 2030. She became the founding director of the Science Fiction Museum and Hall of Fame in Seattle in 2004 after leaving NASA, and to this day, she enjoys checking out pop culture portrayals of Mars landings – even if they're not always accurate.
After the novel The Martian was published in 2011, which later was adapted into the hit film starring Matt Damon, Shirley phoned author Andy Weir: "You've got a major mistake in here. It says there's a storm that tries to blow the rocket over. But actually, the Mars atmosphere is so thin, it would never blow a rocket over!"
Fearlessly speaking her mind and seeking the stars helped Donna Shirley make history. However, a 2019 Washington Post story noted: "Women make up only about a third of NASA's workforce. They comprise just 28 percent of senior executive leadership positions and are only 16 percent of senior scientific employees." Whether it's traveling to Mars or trending toward gender equality, we've still got a long way to go.
Announcing March Event: "COVID Vaccines and the Return to Life: Part 1"
Leading medical and scientific experts will discuss the latest developments around the COVID-19 vaccines at our March 11th event.
EVENT INFORMATION
DATE:
Thursday, March 11th, 2021 at 12:30pm - 1:45pm EST
On the one-year anniversary of the global declaration of the pandemic, this virtual event will convene leading scientific and medical experts to discuss the most pressing questions around the COVID-19 vaccines. Planned topics include the effect of the new circulating variants on the vaccines, what we know so far about transmission dynamics post-vaccination, how individuals can behave post-vaccination, the myths of "good" and "bad" vaccines as more alternatives come on board, and more. A public Q&A will follow the expert discussion.
CONTACT:
kira@goodinc.com
LOCATION:
Zoom webinar
SPEAKERS:

Dr. Paul Offit speaking at Communicating Vaccine Science.
commons.wikimedia.orgDr. Paul Offit, M.D., is the director of the Vaccine Education Center and an attending physician in infectious diseases at the Children's Hospital of Philadelphia. He is a co-inventor of the rotavirus vaccine for infants, and he has lent his expertise to the advisory committees that review data on new vaccines for the CDC and FDA.

Dr. Monica Gandhi
UCSF Health
Dr. Monica Gandhi, M.D., MPH, is Professor of Medicine and Associate Division Chief (Clinical Operations/ Education) of the Division of HIV, Infectious Diseases, and Global Medicine at UCSF/ San Francisco General Hospital.

Dr. Onyema Ogbuagu, MBBCh, FACP, FIDSA
Yale Medicine
Dr. Onyema Ogbuagu, MBBCh, is an infectious disease physician at Yale Medicine who treats COVID-19 patients and leads Yale's clinical studies around COVID-19. He ran Yale's trial of the Pfizer/BioNTech vaccine.

Dr. Eric Topol
Dr. Topol's Twitter
Dr. Eric Topol, M.D., is a cardiologist, scientist, professor of molecular medicine, and the director and founder of Scripps Research Translational Institute. He has led clinical trials in over 40 countries with over 200,000 patients and pioneered the development of many routinely used medications.
REGISTER NOW
This event is the first of a four-part series co-hosted by LeapsMag, the Aspen Institute Science & Society Program, and the Sabin–Aspen Vaccine Science & Policy Group, with generous support from the Gordon and Betty Moore Foundation and the Howard Hughes Medical Institute.

Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.