Tiny, tough “water bears” may help bring new vaccines and medicines to sub-Saharan Africa
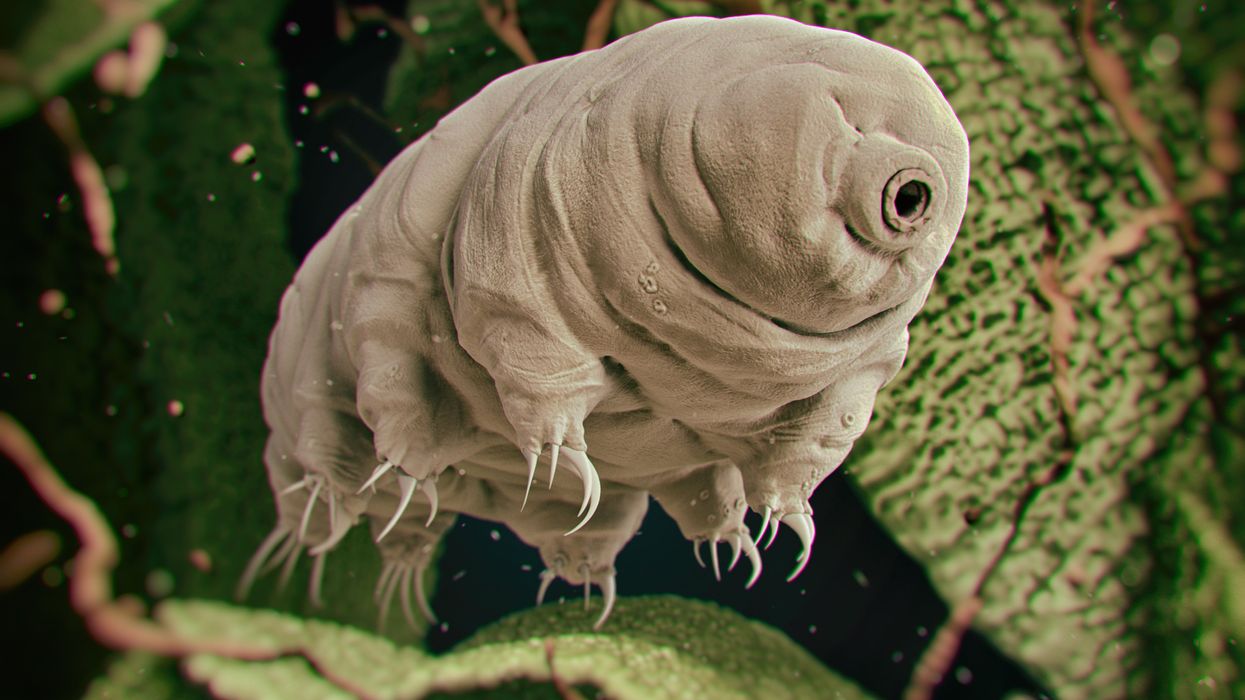
Tardigrades can completely dehydrate and later rehydrate themselves, a survival trick that scientists are harnessing to preserve medicines in hot temperatures.
Microscopic tardigrades, widely considered to be some of the toughest animals on earth, can survive for decades without oxygen or water and are thought to have lived through a crash-landing on the moon. Also known as water bears, they survive by fully dehydrating and later rehydrating themselves – a feat only a few animals can accomplish. Now scientists are harnessing tardigrades’ talents to make medicines that can be dried and stored at ambient temperatures and later rehydrated for use—instead of being kept refrigerated or frozen.
Many biologics—pharmaceutical products made by using living cells or synthesized from biological sources—require refrigeration, which isn’t always available in many remote locales or places with unreliable electricity. These products include mRNA and other vaccines, monoclonal antibodies and immuno-therapies for cancer, rheumatoid arthritis and other conditions. Cooling is also needed for medicines for blood clotting disorders like hemophilia and for trauma patients.
Formulating biologics to withstand drying and hot temperatures has been the holy grail for pharmaceutical researchers for decades. It’s a hard feat to manage. “Biologic pharmaceuticals are highly efficacious, but many are inherently unstable,” says Thomas Boothby, assistant professor of molecular biology at University of Wyoming. Therefore, during storage and shipping, they must be refrigerated at 2 to 8 degrees Celsius (35 to 46 degrees Fahrenheit). Some must be frozen, typically at -20 degrees Celsius, but sometimes as low -90 degrees Celsius as was the case with the Pfizer Covid vaccine.
For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
The costly cold chain
The logistics network that ensures those temperature requirements are met from production to administration is called the cold chain. This cold chain network is often unreliable or entirely lacking in remote, rural areas in developing nations that have malfunctioning electrical grids. “Almost all routine vaccines require a cold chain,” says Christopher Fox, senior vice president of formulations at the Access to Advanced Health Institute. But when the power goes out, so does refrigeration, putting refrigerated or frozen medical products at risk. Consequently, the mRNA vaccines developed for Covid-19 and other conditions, as well as more traditional vaccines for cholera, tetanus and other diseases, often can’t be delivered to the most remote parts of the world.
To understand the scope of the challenge, consider this: In the U.S., more than 984 million doses of Covid-19 vaccine have been distributed so far. Each one needed refrigeration that, even in the U.S., proved challenging. Now extrapolate to all vaccines and the entire world. For Covid, fewer than 73 percent of the global population received even one dose. The need for refrigerated or frozen handling was partially to blame.
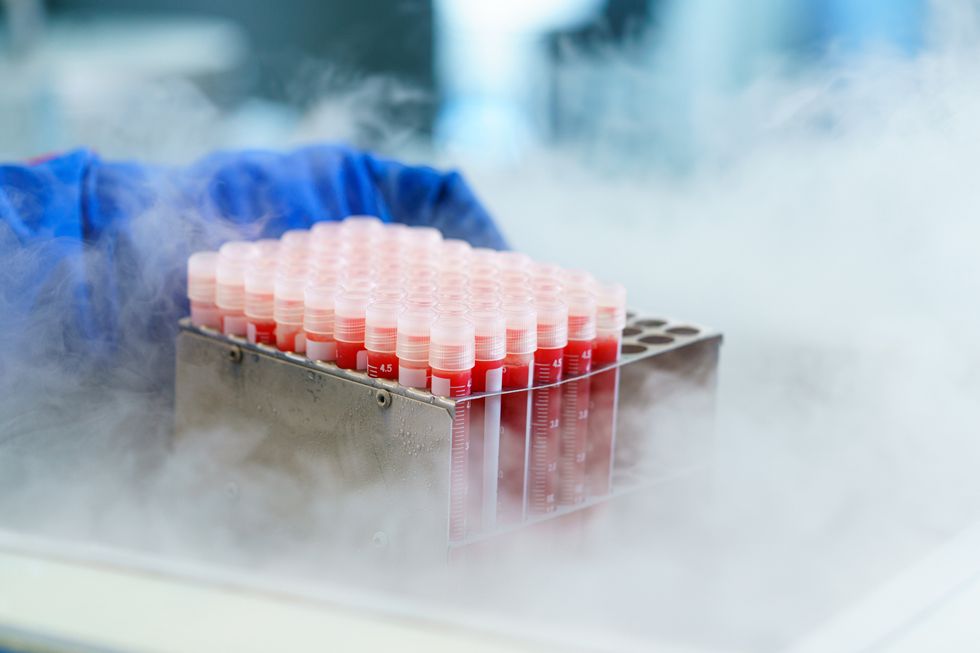
Globally, the cold chain packaging market is valued at over $15 billion and is expected to exceed $60 billion by 2033.
Adobe Stock
Freeze-drying, also called lyophilization, which is common for many vaccines, isn’t always an option. Many freeze-dried vaccines still need refrigeration, and even medicines approved for storage at ambient temperatures break down in the heat of sub-Saharan Africa. “Even in a freeze-dried state, biologics often will undergo partial rehydration and dehydration, which can be extremely damaging,” Boothby explains.
The cold chain is also very expensive to maintain. The global pharmaceutical cold chain packaging market is valued at more than $15 billion, and is expected to exceed $60 billion by 2033, according to a report by Future Market Insights. This cost is only expected to grow. According to the consulting company Accenture, the number of medicines that require the cold chain are expected to grow by 48 percent, compared to only 21 percent for non-cold-chain therapies.
Tardigrades to the rescue
Tardigrades are only about a millimeter long – with four legs and claws, and they lumber around like bears, thus their nickname – but could provide a big solution. “Tardigrades are unique in the animal kingdom, in that they’re able to survive a vast array of environmental insults,” says Boothby, the Wyoming professor. “They can be dried out, frozen, heated past the boiling point of water and irradiated at levels that are thousands of times more than you or I could survive.” So, his team is gradually unlocking tardigrades’ survival secrets and applying them to biologic pharmaceuticals to make them withstand both extreme heat and desiccation without losing efficacy.
Boothby’s team is focusing on blood clotting factor VIII, which, as the name implies, causes blood to clot. Currently, Boothby is concentrating on the so-called cytoplasmic abundant heat soluble (CAHS) protein family, which is found only in tardigrades, protecting them when they dry out. “We showed we can desiccate a biologic (blood clotting factor VIII, a key clotting component) in the presence of tardigrade proteins,” he says—without losing any of its effectiveness.
The researchers mixed the tardigrade protein with the blood clotting factor and then dried and rehydrated that substance six times without damaging the latter. This suggests that biologics protected with tardigrade proteins can withstand real-world fluctuations in humidity.
Furthermore, Boothby’s team found that when the blood clotting factor was dried and stabilized with tardigrade proteins, it retained its efficacy at temperatures as high as 95 degrees Celsius. That’s over 200 degrees Fahrenheit, much hotter than the 58 degrees Celsius that the World Meteorological Organization lists as the hottest recorded air temperature on earth. In contrast, without the protein, the blood clotting factor degraded significantly. The team published their findings in the journal Nature in March.
Although tardigrades rarely live more than 2.5 years, they have survived in a desiccated state for up to two decades, according to Animal Diversity Web. This suggests that tardigrades’ CAHS protein can protect biologic pharmaceuticals nearly indefinitely without refrigeration or freezing, which makes it significantly easier to deliver them in locations where refrigeration is unreliable or doesn’t exist.
The tricks of the tardigrades
Besides the CAHS proteins, tardigrades rely on a type of sugar called trehalose and some other protectants. So, rather than drying up, their cells solidify into rigid, glass-like structures. As that happens, viscosity between cells increases, thereby slowing their biological functions so much that they all but stop.
Now Boothby is combining CAHS D, one of the proteins in the CAHS family, with trehalose. He found that CAHS D and trehalose each protected proteins through repeated drying and rehydrating cycles. They also work synergistically, which means that together they might stabilize biologics under a variety of dry storage conditions.
“We’re finding the protective effect is not just additive but actually is synergistic,” he says. “We’re keen to see if something like that also holds true with different protein combinations.” If so, combinations could possibly protect against a variety of conditions.
Commercialization outlook
Before any stabilization technology for biologics can be commercialized, it first must be approved by the appropriate regulators. In the U.S., that’s the U.S. Food and Drug Administration. Developing a new formulation would require clinical testing and vast numbers of participants. So existing vaccines and biologics likely won’t be re-formulated for dry storage. “Many were developed decades ago,” says Fox. “They‘re not going to be reformulated into thermo-stable vaccines overnight,” if ever, he predicts.
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits.
Instead, this technology is most likely to be used for the new products and formulations that are just being created. New and improved vaccines will be the first to benefit. Good candidates include the plethora of mRNA vaccines, as well as biologic pharmaceuticals for neglected diseases that affect parts of the world where reliable cold chain is difficult to maintain, Boothby says. Some examples include new, more effective vaccines for malaria and for pathogenic Escherichia coli, which causes diarrhea.
Tallying up the benefits
Extending stability outside the cold chain, even for a few days, can have profound health, environmental and economic benefits. For instance, MenAfriVac, a meningitis vaccine (without tardigrade proteins) developed for sub-Saharan Africa, can be stored at up to 40 degrees Celsius for four days before administration. “If you have a few days where you don’t need to maintain the cold chain, it’s easier to transport vaccines to remote areas,” Fox says, where refrigeration does not exist or is not reliable.
Better health is an obvious benefit. MenAfriVac reduced suspected meningitis cases by 57 percent in the overall population and more than 99 percent among vaccinated individuals.
Lower healthcare costs are another benefit. One study done in Togo found that the cold chain-related costs increased the per dose vaccine price up to 11-fold. The ability to ship the vaccines using the usual cold chain, but transporting them at ambient temperatures for the final few days cut the cost in half.
There are environmental benefits, too, such as reducing fuel consumption and greenhouse gas emissions. Cold chain transports consume 20 percent more fuel than non-cold chain shipping, due to refrigeration equipment, according to the International Trade Administration.
A study by researchers at Johns Hopkins University compared the greenhouse gas emissions of the new, oral Vaxart COVID-19 vaccine (which doesn’t require refrigeration) with four intramuscular vaccines (which require refrigeration or freezing). While the Vaxart vaccine is still in clinical trials, the study found that “up to 82.25 million kilograms of CO2 could be averted by using oral vaccines in the U.S. alone.” That is akin to taking 17,700 vehicles out of service for one year.
Although tardigrades’ protective proteins won’t be a component of biologic pharmaceutics for several years, scientists are proving that this approach is viable. They are hopeful that a day will come when vaccines and biologics can be delivered anywhere in the world without needing refrigerators or freezers en route.
How a Nobel-Prize Winner Fought Her Family, Nazis, and Bombs to Change our Understanding of Cells Forever
Rita Levi-Montalcini survived the Nazis and eventually won a Nobel Prize for her work to understand why certain cells grow so quickly.
When Rita Levi-Montalcini decided to become a scientist, she was determined that nothing would stand in her way. And from the beginning, that determination was put to the test. Before Levi-Montalcini became a Nobel Prize-winning neurobiologist, the first to discover and isolate a crucial chemical called Neural Growth Factor (NGF), she would have to battle both the sexism within her own family as well as the racism and fascism that was slowly engulfing her country
Levi-Montalcini was born to two loving parents in Turin, Italy at the turn of the 20th century. She and her twin sister, Paola, were the youngest of the family's four children, and Levi-Montalcini described her childhood as "filled with love and reciprocal devotion." But while her parents were loving, supportive and "highly cultured," her father refused to let his three daughters engage in any schooling beyond the basics. "He loved us and had a great respect for women," she later explained, "but he believed that a professional career would interfere with the duties of a wife and mother."
At age 20, Levi-Montalcini had finally had enough. "I realized that I could not possibly adjust to a feminine role as conceived by my father," she is quoted as saying, and asked his permission to finish high school and pursue a career in medicine. When her father reluctantly agreed, Levi-Montalcini was ecstatic: In just under a year, she managed to catch up on her mathematics, graduate high school, and enroll in medical school in Turin.
By 1936, Levi-Montalcini had graduated medical school at the top of her class and decided to stay on at the University of Turin as a research assistant for histologist and human anatomy professor Guiseppe Levi. Levi-Montalcini started studying nerve cells and nerve fibers – the tiny, slender tendrils that are threaded throughout our nerves and that determine what information each nerve can transmit. But it wasn't long before another enormous obstacle to her scientific career reared its head.
Science Under a Fascist Regime
Two years into her research assistant position, Levi-Montalcini was fired, along with every other "non-Aryan Italian" who held an academic or professional career, thanks to a series of antisemitic laws passed by Italy's then-leader Benito Mussolini. Forced out of her academic position, Levi-Montalcini went to Belgium for a fellowship at a neurological institute in Brussels – but then was forced back to Turin when the German army invaded.
Levi-Montalcini decided to keep researching. She and Guiseppe Levi built a makeshift lab in Levi-Montalcini's apartment, borrowing chicken eggs from local farmers and using sewing needles to dissect them. By dissecting the chicken embryos from her bedroom laboratory, she was able to see how nerve fibers formed and died. The two continued this research until they were interrupted again – this time, by British air raids. Levi-Montalcini fled to a country cottage to continue her research, and then two years later was forced into hiding when the German army invaded Italy. Levi-Montalcini and her family assumed different identities and lived with non-Jewish friends in Florence to survive the Holocaust. Despite all of this, Levi-Montalcini continued her work, dissecting chicken embryos from her hiding place until the end of the war.
"The discovery of NGF really changed the world in which we live, because now we knew that cells talk to other cells, and that they use soluble factors. It was hugely important."
A Post-War Discovery
Several years after the war, when Levi-Montalcini was once again working at the University of Turin, a German embryologist named Viktor Hamburger invited her to Washington University in St. Louis. Hamburger was impressed by Levi-Montalcini's research with her chicken embryos, and secured an opportunity for her to continue her work in America. The invitation would "change the course of my life," Levi-Montalcini would later recall.
During her fellowship, Montalcini grew tumors in mice and then transferred them to chick embryos in order to see how it would affect the chickens. To her surprise, she noticed that introducing the tumor samples would cause nerve fibers to grow rapidly. From this, Levi-Montalcini discovered and was able to isolate a protein that she determined was able to cause this rapid growth. She later named this Nerve Growth Factor, or NGF.
From there, Levi-Montalcini and her team launched new experiments to test NGF, injecting it and repressing it to see the effect it had in a test subject's body. When the team injected NGF into embryonic mice, they observed nerve growth, as well as the mouse pups developing faster – their eyes opening earlier and their teeth coming in sooner – than the untreated group. When the team purified the NGF extract, however, it had no effect, leading the team to believe that something else in the crude extract of NGF was influencing the growth of the newborn mice. Stanley Cohen, Levi-Montalcini's colleague, identified another growth factor called EGF – epidermal growth factor – that caused the mouse pups' eyes and teeth to grow so quickly.
Levi-Montalcini continued to experiment with NGF for the next several decades at Washington University, illuminating how NGF works in our body. When Levi-Montalcini injected newborn mice with an antiserum for NGF, for example, her team found that it "almost completely deprived the animals of a sympathetic nervous system." Other experiments done by Levi-Montalcini and her colleagues helped show the role that NGF plays in other important biological processes, such as the regulation of our immune system and ovulation.
"The discovery of NGF really changed the world in which we live, because now we knew that cells talk to other cells, and that they use soluble factors. It was hugely important," said Bill Mobley, Chair of the Department of Neurosciences at the University of California, San Diego School of Medicine.
Her Lasting Legacy
After years of setbacks, Levi-Montalcini's groundbreaking work was recognized in 1986, when she was awarded the Nobel Prize in Medicine for her discovery of NGF (Cohen, her colleague who discovered EGF, shared the prize). Researchers continue to study NGF even to this day, and the continued research has been able to increase our understanding of diseases like HIV and Alzheimer's.
Levi-Montalcini never stopped researching either: In January 2012, at the age of 102, Levi-Montalcini published her last research paper in the journal PNAS, making her the oldest member of the National Academy of Science to do so. Before she died in December 2012, she encouraged other scientists who would suffer setbacks in their careers to keep pursuing their passions. "Don't fear the difficult moments," Levi-Montalcini is quoted as saying. "The best comes from them."
60-Second Video: How Safe Are the COVID-19 Vaccines?
Comparing the Risks of COVID-19 vs. the Risks of the Vaccines:
Kira Peikoff was the editor-in-chief of Leaps.org from 2017 to 2021. As a journalist, her work has appeared in The New York Times, Newsweek, Nautilus, Popular Mechanics, The New York Academy of Sciences, and other outlets. She is also the author of four suspense novels that explore controversial issues arising from scientific innovation: Living Proof, No Time to Die, Die Again Tomorrow, and Mother Knows Best. Peikoff holds a B.A. in Journalism from New York University and an M.S. in Bioethics from Columbia University. She lives in New Jersey with her husband and two young sons. Follow her on Twitter @KiraPeikoff.

